Hypoxia Detection Assays
Measurement of Oxygen Levels in Tissues and Cells
Defined as an oxygen deficiency in tissues, hypoxia is a hallmark of primary tumors that occurs due to impaired vascularization and the increased respiratory demand of rapidly proliferating cancer cells. With blood capiliaries concentrated at the periphery, the tumor microenvironment generates an oxygen gradient with low [O2] near the necrotic core and high [O2] at the tumor surface (Figure 1). Hypoxia affects many aspects of metastatic disease, including cell proliferation, metabolic capacity, immune response, and resistance to chemotherapeutic intervention. At the cellular level, hypoxic responses are mediated by hypoxia-inducible (HIF) transcription factors, which regulate gene expression driving the adaptation of resident cells. Unraveling the underlying mechanisms of hypoxic responses is critical to the development of therapeutics targeting tumor progression. We offer a variety of hypoxia detection kits and assays to measure oxygen levels in both fixed and live cells and tissues.

Figure 1.The hypoxic tumor microenvironment. Solid tumors rapidly outgrow their blood supply, leaving tumor regions with oxygen concentrations significantly lower than those found in healthy tissues. Hypoxic conditions lead to cancer cells with increased mutation rates, drug-efflux, and evasion of apoptosis, as well as decreased overall cell proliferation, drug solubility, and secretion of soluble cytokines and nutrients.
Measuring Oxygen Levels in Fixed Tissues and Cells
Several markers for the detection of hypoxia by imaging have been identified and are widely used in research. Developed at the University of Pennsylvania by Koch and Evans, EF5 (a 2-nitroimidazole-based molecule) selectively identifies hypoxic cells. Upon injection into fixed tissues, EF5 selectively binds to hypoxic cells and forms adducts. A fluorescently conjugated mouse monoclonal antibody can then be used to selectively bind and identify EF5 adducts, signaling hypoxic environments (Figure 2). We offer complete ready-to-use kits and standalone EF5 hypoxia detection reagents, including conjugated EF5 antibodies, buffers, and EF5 compounds.
Key advantages of EF5 Hypoxia Detection Kit:
- Unlike pimonidazole, single lipophilic EF5 form facilitates rapid and even tissue distribution
- Calibration of EF5 binding images provides quantitative pO2 values for each cell
- Highly cited in over 2000 publications
Materials
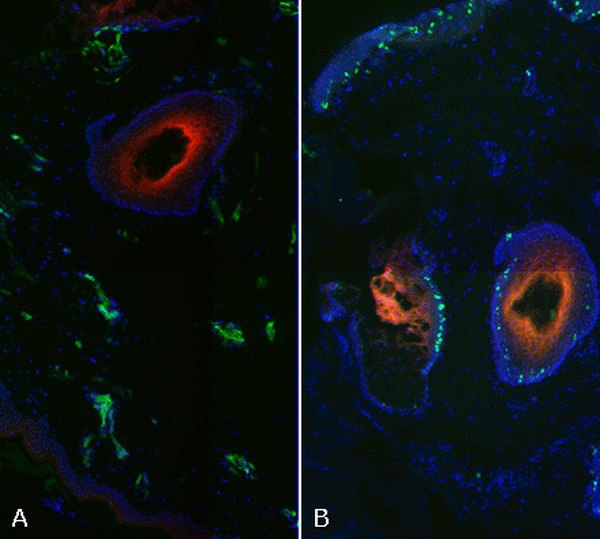
Figure 2. Hypoxia detection in fixed tissue.Human skin demonstrating the relationship between EF5 binding (red, Figures A & B) and blood vessels (green, CD31; Figure A) or proliferating cells (green; Ki67 - Figure B).
Live Cell Hypoxia Detection
Hypoxia in living tissue is correlated with several pathologies, including cancer and ischemia. Existing hypoxia detection probes, such as pimonidazole and EF5, require cell fixation followed by immunostaining. BioTracker 520 Green Hypoxia Dye is a fluorescent imaging probe for the detection of hypoxia in living cells (Figure 3). With sensitivity comparable to pimonidazole, BioTracker hypoxia dye can be used in live cell fluorescence imaging and flow cytometry applications (Figures 4 and 5).
Live Cell Imaging of Hypoxia in Cancer Cells using the CellASIC® ONIX2 Microfluidic System
Materials
Figure 3. BioTracker hypoxia dye mechanism.Reductive cleavage of the azo base in the BioTracker 520 Green Hypoxia Dye occurs under hypoxic conditions generating 2Me RG which emits bright green fluorescence. Lower oxygen levels (more severe hypoxic conditions) lead to greater fluorescence intensities.
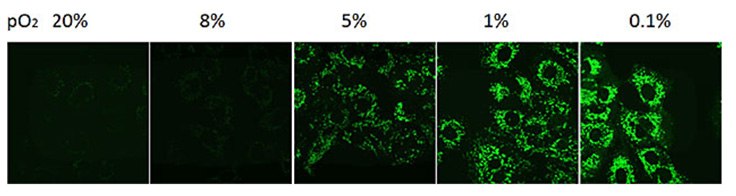
Figure 4. Measurement of hypoxia in cancer cells.Fluorescent imaging of live A549 cells subjected to decreasing oxygen concentrations using BioTracker 520 Green Hypoxia Dye.
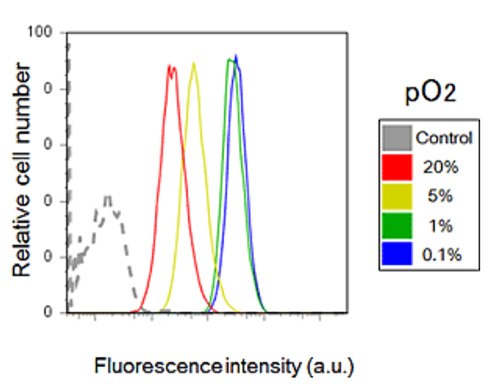
Figure 5. Hypoxia detection using flow cytometry.A549 cells were analyzed by flow cytometry after incubation for 6 hours under various oxygen concentrations and stained with 1 μM BioTracker 520 Green Hypoxia Dye. Fluorescent intensity increased as oxygen concentration decreased, indicating that the dye is a viable probe for hypoxia detection using flow cytometry.
Microfluidic Control of Hypoxic Microenvironments
The CellASIC® ONIX2 imaging platform controls media perfusion, gas exchange, and temperature in a single device for automated, long-term cell culture with continuous live-cell imaging.This innovative design permits cell exposure to different environmental conditions via pressurized flow channels and control of culturing parameters, with user-specified changes in media source and flow rate.
The culture unit consists of a microfluidic plate and environmental control system (Figure 6). Plates are constructed with gas-permeable materials and aeration channels, minimizing diffusive effects. Micro-volume cultures allow for more precise chemo-temporal and spatial control, including rapid changes to experimental conditions. The controller regulates pressurized cell loading, programmable media perfusion, temperature, and gas levels. Due to its compact size and high optical clarity, the plate can be paired with most inverted microscopes for automated dynamic analysis. When in combination with fluorescent visualization, ONIX2 can be used to monitor changes in labeled protein expression (Figures 7 and 8) and to discriminate and analyze multiple cell types simultaneously.
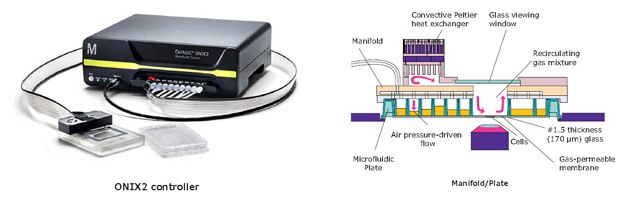
Figure 6.Diagram of CellASIC


Figure 7. Chemoresistance changes parallel HIF1α protein levels.A) During hypoxic conditioning and normoxic re-establishment, cells were fixed and stained to identify total cells and hypoxia-responsive fraction. B) Hif1α levels correlated with changes in chemo-resistance, peaking at 4 hours with extended hypoxic exposure. Normalized fluorescence intensity of total cells (DAPI) and hypoxia-responsive fraction (Hif1α) (both images 20X).
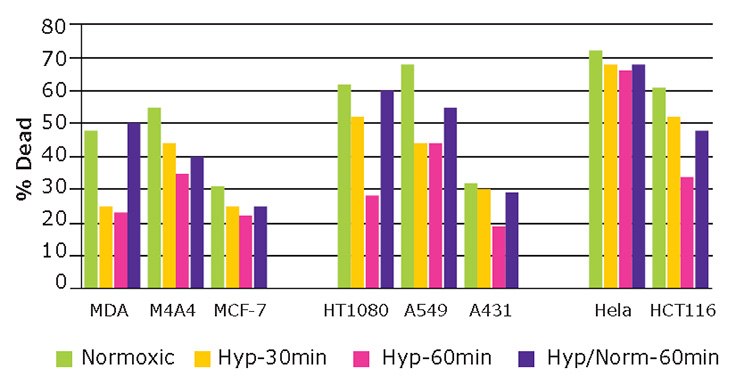
Figure 8. Hypoxic responses of cancer cell lines.Eight cancer cell lines (MDA-MB-231, M4A4, MCF7, HT1080, A549, A431, HeLa, HCT116) from diverse tissue origins and of different metastatic potential, were preconditioned to hypoxia (with or without re-establishing standard oxygen levels) and exposed to the cytotoxic agent staurosporine(STX).
Conclusion
Hypoxia is known to impact a multitude of tumor cell functions and processes, including cell proliferation, resistance to cytotoxic agents, cell death mechanisms, and metastatic potential. Further elucidation of hypoxia mechanisms and processes will allow researchers to prevent cancer progression and develop more effective and robust chemotherapeutics. Regardless of your experimental approach, we are committed to helping you solve the toughest problems in life science by accelerating access to innovative methods and materials. From fixed tissues to live cell imaging, we have the reagents, tools, and instruments to accelerate your research into hypoxia in cancer and other disease states.
Reference
To continue reading please sign in or create an account.
Don't Have An Account?