Settle Plates for Active and Passive Air Monitoring
Passive and active air sampling are essential methods for monitoring airborne microorganisms in controlled environments, particularly in the pharmaceutical and cosmetics industries.
Passive air monitoring is usually performed by exposing the settle plates to the air for a given time and then incubating them to allow visible colonies to develop and be counted. The viable biological particles settle onto the plate's surface, allowing for qualitative analysis of airborne microorganisms once the plates are incubated.
Active air monitoring with settle plates or contact plates utilizes a microbial air sampler to draw a predetermined volume of air over a collection medium, such as agar plates. The plate is then removed from the air sampler and directly incubated, allowing visible colonies to develop and subsequently be counted. The number of visible colonies provides an estimate of the number of colony-forming units in the sampled air.
Section Overview
- ICR and ICRplus Gamma-Irradiated Settle Plates
- Non-Lockable ICR Settle Plates for Isolators and Critical Cleanrooms
- Lockable ICRPlus Settle Plates for Isolators and Critical Cleanrooms
- Non-Irradiated Settle Plates (LI Settle Plates) for Less Critical Areas
- Explore Culture Media Products for Active and Passive Air Monitoring
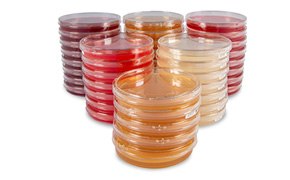
We offer high-quality settle plates, including the gamma irradiated ICR and ICRplus plates, that are specifically designed for air monitoring in critical and less critical clean rooms. Our settle plates meet all relevant international standards and regulations, including EU cGMP, FDA Aseptic Guidance and USP 34<1116>. Our settle plates are easy-to-use, enabling continuous and efficient monitoring for microorganisms during production process.
ICR and ICRplus settle plates are designed to meet the demands of environmental monitoring in isolators and cleanrooms. The plates are gamma-irradiated and triple bagged to minimize the risk of cross-contamination, making them ideal for active air monitoring with our MAS Air Samplers and for passive microbial air sampling in isolators and clean rooms.
Our settle plates contain either Tryptic Soy Agar (TSA), Sabouraud Dextrose Agar (SDA) or a non-animal, vegetable peptone medium to reduce the risk of BSE/TSE contamination, all prepared in accordance with the recommendations of the European and US pharmacopeias. We offer Sabouraud Dextrose Agar formulations in pink plates to facilitate clear and easy differentiation of TSA and SDA.
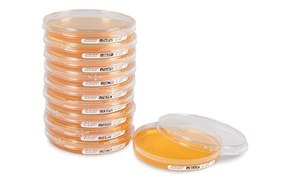
Figure 1.Tripled bagged and gamma irradiated ICR and ICRplus settle plates
The plates are supplemented with neutralizers to inactivate a broad range of disinfectants and β-lactam antibiotics. To minimize the risk of contamination during transportation of the samples from a sterile to a non-classified environment, we provide gamma-sterilized zip lock bags suitable for contact and settle plates.
Additionally, the plates are available in both non-lockable ICR and lockable ICRplus configurations.
Non-lockable ICR settle plates for isolators and critical cleanrooms
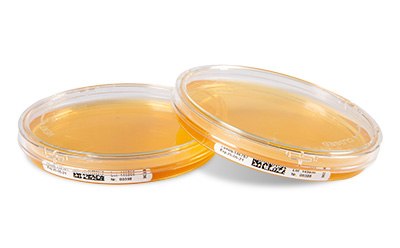
Figure 2.Lockable ICRPlus settle plates
In addition to all the above features and benefits, our ICRPlus settle plates combine the high-quality features of standard ICR Settle Plates with the new two-way closure system (CLOSED- or VENT-position) to:
- Increase safety during transport between the sampling area and laboratory/incubation
- Expand incubation for the detection of anaerobic and microaerophilic microorganisms
NON-IRRADIATED SETTLE PLATES (LI SETTLE PLATES) for Less Critical Areas
Our range of non-irradiated settle plates is designed for use in non-controlled and less critically controlled cleanrooms. They can be used for passive and active air monitoring, as well as glove prints. Each set contains ten plates, which are single-bagged.
Single-bagged long (L) incubation (I) settle plates for environmental monitoring in less critical areas are ideal for monitoring less critical cleanroom areas, such as grade C, D, and E, or non-specified environments. Their high-filling volume of 30 mL in 90 mm settle plates reduces the percentage of water loss during the air monitoring procedures.

Figure 3.TSA and pink coloured SDA ICR plates
LI Settle Plates feature a higher than standard filling volume of 30 mL to compensate for water loss during extended incubation periods. These Settle Plates contain either Tryptic Soy Agar (TSA) or Sabouraud Dextrose Agar (SDA), which is ideally suited for the growth of yeast and molds are prepared according to the recommendations of the European and US pharmacopeias. They are supplemented with neutralizers such as Lecithin (L), Tween 80 (T), histidine (H) and sodium thiosulfate (Th) to inactivate a wide range of disinfectants. Additionally, to suppress bacterial growth, SDA media is made available with chloramphenicol.
Tryptic Soy Agar
Sabouraud Dextrose Agar
CHOCOLATE AGAR +LTH - SDV
Media and Accessories
To continue reading please sign in or create an account.
Don't Have An Account?