Environmental Monitoring and Aseptic Process Simulation
Patient safety is crucial, thus pharmaceutical products are manufactured under stringent controls. Microbial monitoring plays a vital role in GMP regulatory compliance, demonstrating that manufacturing processes, particularly in aseptic production, are well-managed.
Explore our comprehensive range of GMP-compliant solutions for microbial monitoring in aseptic pharmaceutical manufacturing, which includes monitoring of surfaces, personnel, and air. We also offer tailored solutions for isolators and RABS to enhance your workflow.
Additionally, we provide both granulated and ready-to-use culture media for media fills and aseptic process simulations, supporting classical environmental monitoring.
Products
Products
Microbial Air Samplers
Our air samplers are based on different principles, enabling technological selection to suit your environmental monitoring needs. We offer products specifically designed for explosion hazard areas, cleanrooms and isolators, and compressed gas monitoring. Our easy-to-use monitoring systems are designed for many environmental monitoring needs.
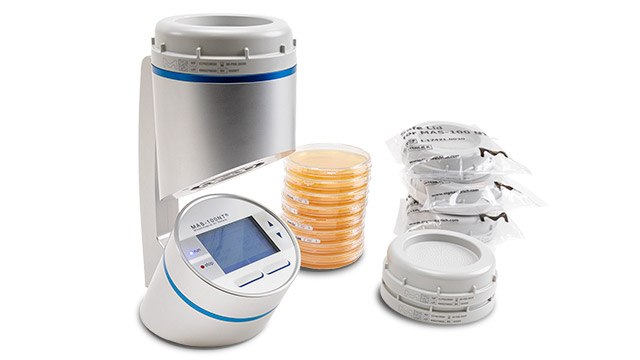
- The new MAS-100 Sirius® portable microbial air sampler enhances active air monitoring in high-grade cleanrooms, making it more reliable and convenient than ever. Designed for a digital environment, this air sampler supports data integrity and full traceability to ensure compliance.
- The MAS-100 VF® active air sampler is specifically tailored for controlled environments. Its compact design and ease of handling features make it ideal for monitoring environment quality.
- The MAS-100 Atmos® compressed gas sampler is simple, safe, and compliant with 21 CFR Part 11, allowing for easy and automatic detection of microbial contamination in gases.
- ICR and ICRplus settle plates features a high filling volume of 30 mL in 90 mm plates. They are gamma-irradiated and triple bagged, making them ideal for both active air monitoring with MAS-100® Air Samplers and passive microbial air sampling in isolators and clean rooms.
Surface and Personnel Monitoring
We provide a wide selection of plates tailored for environmental monitoring in isolators and critical cleanrooms, including the non-lockable ICR and lockable ICRplus Contact Plates with TSA + LTHTh.
- ICR Contact plates are designed to minimize the risk of contamination as they are produced in clean rooms. The lockable ICRplus/RTplus versions can be safely transported for incubation under aerobic, microaerophilic, or anaerobic conditions.
- ICR Swabs are designed for absence-presence testing on dry surfaces in isolators and cleanrooms that are challenging to access for surface and personnel monitoring. Key features include:
Isolator and RABS solutions
Isolators and RABS are designed to eliminate human contact during controlled manufacturing.
- The MAS-100 Iso MH® microbial air sampler is engineered for the highest collection efficiency and safety in isolators. For only a single sampling point in your Grade A area, we offer the MAS-100 Iso NT® air sampler.
- IsoBag® rapid transfer solution enables safe and rapid culture media transfer into isolators and critical areas. The IsoBag® solution expedites environmental monitoring in aseptic production isolators by providing ready-to-use gamma-irradiated contact or settle plates for immediate use.
Culture Media for Aseptic Process Simulation
Our irradiated, triple-wrapped granulated media or ready-to-use media alleviate concerns about compromising validated processes during media fill trials.
- Granulated culture media, carefully formulated, enables pharmaceutical manufacturers to conduct efficient media fill trials with minimal risks.
- Ready-to-use culture media, such as Tryptic Soy Broth (TSB) and Vegetable Peptone Broth (VPB), undergo sterilization by autoclave and a two-step pre-filtration process, ensuring their quality.
Related Resources
- Article: Ensuring Data Integrity in Environmental Monitoring
Learn why data integrity is crucial for environmental monitoring.
- Article: A Guide to Buying the Right Microbial Air Sampler
Compare features and selection criteria to consider when you choose an air sampler.
- Brochure: MAS-100® Air Samplers
Convenient monitoring of compressed gas: with its various operation modes, the MAS-100 Atmos® instrument can adapt to different workflows.
- Data Sheet: Our Services Portfolio Supporting the MAS-100® Family
Discover our services portfolio supporting the MAS-100® family for active air monitoring.
- A solution for safe transfer of materials
Ease safe material transfer into controlled areas, such as cleanrooms and isolators.
- Aseptic Process Simulation in the Pharmaceutical Industry
High quality granulated and ready-to-use culture media irradiated for accurate and reliable aseptic process simulations.
- Study: Environmental Sample Pre Storage on Counting Results
This study reviews data to demonstrate if pre-storage has any impact on the recovery rates and growth promotion of selected microorganisms.
- Settle Plates for Active and Passive Air Monitoring
High quality settle plates, including irradiated ICR and ICRplus plates and specifically designed for air monitoring in critical areas.
- Contact Plates and Swabs for Personnel and Surface Monitoring
Ready-to-use plates and swabs for environmental monitoring in critical and less critical areas.
- Study: Detection of Low Bacterial Contamination from Neoprene® Gloves
This article shows the suitability of ICR swabs to detect low numbers of different bacterial test strains from Neoprene® gloves.
- Brochure: Environmental Monitoring Solutions for Pharmaceutical Industry
GMP compliant solutions for process monitoring in pharmaceutical manufacturing.
To continue reading please sign in or create an account.
Don't Have An Account?