Stimuli-Responsive Materials as Intelligent Drug Delivery Systems
Materials science has revolutionized the central paradigm of drug delivery, especially for cancer therapeutics, such that the physicochemical properties of the delivery system can be customized to develop so-called smart or intelligent systems that can deliver the therapeutic molecule on-demand. Much of the advances in the design of materials for drug delivery has been inspired by a growing understanding of tumor microenvironments and the exploitation of the subtle differences in the tumor bio-milieu. Cancer is a complex disease involving many different cell types, extracellular matrices, immune factors, signaling molecules, and physiological phenomena. The significant diversity of the cell types involved in cancer poses a significant challenge for targeting a tumor with a therapeutic drug. Acquired multidrug resistance (MDR) to existing chemotherapies further compounds the problem, inevitably leading to poor clinical outcomes. The tumor microenvironment is remarkably diverse; it is characterized by a variety of characteristics, including abnormal tumor vasculature, absence of lymphatic drainage, hypoxia, lower pH gradient, redox environment, high interstitial pressure, and high protease activity.1 At the cellular level, the tumor is comprised not only of cancer cells but also a diverse population that includes stromal cells, endothelial cells, components of immune cells, and cancer stem cells (CSCs).2 These heterogeneities within the tumor impart survival advantages and promote tumor growth, and also help to progress and disseminate the disease to distant sites. Alternatively, subtle differences in tumor physiology can be used to stimulate a response using a smart polymer system, enabling the design of therapeutic strategies with tumor specificity.
Internally Regulated Systems
Internally regulated vectors (also known as self-regulated or closed-loop systems) respond to a stimulus from within the body such as pH, redox, presence of proteases, or other factors to regulate drug release. Change in the bio-milieu at the diseased site triggers a chemical or physical change in the delivery system, which leads to the release of the payload. The release profile is entirely dependent on the physiological status of the site of the disease and cannot be modulated externally.
pH-Responsive Systems
pH-responsive systems take advantage of the significant variations in pH to stimulate localized drug delivery to different regions of the body such as the gastrointestinal tract, tumor microenvironment, or to the endosomal/lysosomal compartments of the cell (Figure 1). Cancer cells prefer aerobic glycolysis as their primary source of energy irrespective of the oxygen concentration. This leads to accumulation of lactate in the tumor microenvironment which lowers the pH in the extracellular matrix. This phenomenon is often referred to as the “Warburg effect”. This not only serves the incessant demand for energy of a rapidly dividing cancer cell but also supplies the essential precursors for other macromolecule biosynthesis.4 pH-responsive vectors are typically designed using polymers that contain ionizable weak acids or weak bases to exploit the acidic microenvironment for controlled drug delivery into the tumors. These materials depend on protonation and deprotonation for the selective solubility in aqueous media. Acrylic acid (AAc) (Prod. No. 147230), methacrylic acid (MAAc) (Prod. No. 155721), maleic anhydride (MA) (Prod. No. M188), N,N-dimethylaminoethyl methacrylate (DMAEMA) and 2-(methacryloyloxy)ethyl dihydrogen phosphate are some examples of weak acids and their derivatives that have been explored while poly(amidoamine) (PAA or PAMAM) is a common example of a polymeric weak base that has been extensively used for the design of pH-responsive delivery vectors. Poly(β-aminoester) (PbAE) is another polymer that possesses strong pH dependent solubility and PbAE has also been used in the design of pH-responsive delivery systems. A comprehensive in vitro and in vivo study using pH-responsive poly(ethylene oxide)-PbAE (PEO-PbAE) copolymer system demonstrated higher apoptosis in MDA-MB-231 breast cancer cells and effectively accumulated into the SKOV3 human ovarian cancer xenograft model.5–7 Several pH-responsive vectors have been suitable for delivery of biologics such as gene, siRNA, miRNA, peptides and proteins as well, which demonstrated the versatility of the delivery system.8,9
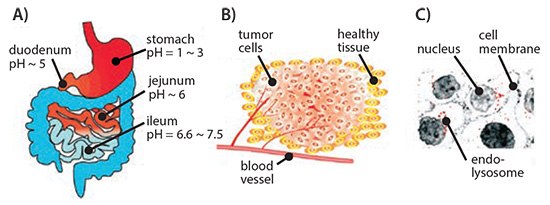
Figure 1.Schematic representation of differential pH environment at the organ (A), tissue (B), and cellular (C) level that could be exploited by pH-responsive drug delivery systems. Reprinted with permission from Reference 8, American Chemical Society 2010.
Redox-responsive Systems
Tumors exhibit characteristic oxidizing extracellular and reducing intracellular environments generating a redox potential that has become the driving force for the development of redox-responsive delivery vectors. Redox-responsive systems tend to lose their structural integrity in response to the significantly higher cytosolic and nucleus concentration of glutathione tripeptide (2–10 mM) compared to the extracellular matrix (2–20 μM). Due to this response, disulfide bonds (S–S) are the most studied redox-sensitive linkage used to develop polymer-, lipidor protein-based delivery systems. Shell shedding copolymers such as PEG-S-S-poly(ε-caprolactone) (PEG-S-S-PCL), PCL-S-S-poly(ethyl ethylene phosphate) (PCL-S-S-PEEP), S-S-PAA-g-PEG, or dextran-S-S-PCL have been successfully employed as redox-responsive delivery systems with faster drug delivery kinetics. This improvement is evident by the in vitro activity of the payload and excellent in vivo tumor growth regression.10 Our group has developed a thiolated gelatin-based protein nanoparticle system that demonstrated tremendous capability in delivering both small molecules and genes for targeting pancreatic cancer cells in pancreatic human adenocarcinoma bearing tumor xenografts.11 In a separate study, these nanoparticles were loaded with plasmid encoding vascular endothelial growth factor (VEGF-1) in an orthotopic MDA-MB-231 human breast adenocarcinoma model which resulted in reduced tumor growth as well as angiogenesis.12 Adopting a layer-by-layer (LbL) assembly approach, poly(vinylpyrrolidone) (PVP) (Prod. No. 81430) coated on silica nanoparticles was used as a sacrificial template to form disulfide crosslinked poly(methacrylic acid) (PMA) capsules for use in the delivery of proteins and peptides for use as vaccines and as small-molecule anticancer drugs.10
Enzyme-responsive Systems
Proteases are an integral part of tumor physiology. Cancer-associated proteases (CAPs) such as matrix metalloproteinases (MMPs), cathepsin, and urokinase plasminogen activators (uPAs) play a crucial role in tumor tissue remodeling and in disease progression, invasion, and dissemination. MMPs have been shown to be overexpressed in a majority of cancers and are generally accepted to be important contributors to cancer progression and invasiveness.13 As a result, enzyme-responsive vectors have been designed with an enzyme-specific peptide in order to trigger delivery when the substrate is degraded by the enzymatic activity within the tumors. One example of these specially designed enzyme-responsive vectors was the local delivery of chemotherapeutic drugs by application of a protease sensitive matrix. Cisplatin conjugated to a protease cleavable peptide CGLDD was further bound to a PEG-diacrylate hydrogel wafer. This approach resulted in prompt drug release in response to the presence of MMP-2 or MMP-9. Dextran-PVGLIG-methotrexate conjugate showed a similar response from the MMPs to release the drug and demonstrated tumor inhibitory effect in vivo.14 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) (Prod. No. 76548) with acetylated dipeptide (N-Ac-AA) along with dioleoyl trimethylammonium propane (DOTAP) and phosphatidylethanolamine (PE) has been used to make nonfusogenic liposomes that turn fusogenic when activated by elastase or proteinase K, thereby improving the intracellular uptake.14
In a similar approach, DOPE functionalized with PEG using a protease responsive linker (e.g., GPLGIAGQ) was blended with disteraoyl phosphatidylcholine (DSPC), cholesteryl chloroformate, and cholesten-5-yloxy-N-(4-((1-imino-2-,β-D-thiogalactosyl ethyl)amino)butyl) formamide (Gal-C4-Chol) to make liposomes with galactose on the surface for targeting hepatic cells. These galactose moieties are shielded by bulky PEG groups, which limit their uptake. However, in the presence of MMP-2, the peptide that links PEG to the liposomal surface is cleaved to expose the targeting ligand, facilitating their rapid uptake.14 In a more recent effort, a cholesterol-anchored graft polymer containing peptide GSGRSAGK (bearing consensus sequence for uPA) and acrylic acid was incorporated in liposomes made using 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, Prod. No. P6354), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, Prod. No. P0763), and cholesterol (47.5:5:47.5 respectively) (Figure 2A). These liposomes with crosslinked polymers showed improved stability with resistance to osmotic swelling or leaking. In the presence of the enzyme, the crosslinking rapidly degraded; this caused drug release through swelling of the liposome (Figure 2B).15 Though the clinical application of these nanovectors is yet to be established, preclinical studies indicate that these systems hold tremendous promise in augmenting therapeutic efficacy of drugs that otherwise show poor bioavailability.
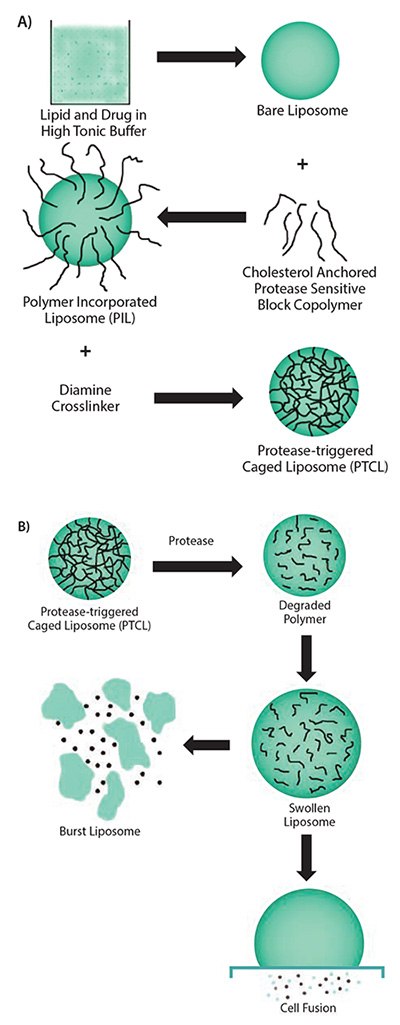
Figure 2.A) Scheme showing the multiple steps involved in the synthesis of uPA-sensitive, polymer-caged liposomes. B) Proposed mechanism of action of the polymer-caged liposomes in response to presence of enzyme. Reprinted with permission from Reference 15, American Chemical Society 2011.
Externally Regulated Systems
Externally regulated systems, also referred to as open-loop systems, are vectors whose drug delivery capability can be governed by a stimulus from outside the body. Since the duration and strength of the external stimulus can be precisely controlled, the drug release profile of these nanovectors can be temporally and spatially controlled to achieve an on-demand supply of the drug at a desired dose. Heat, light, sound, magnetic, and electrical stimuli have all been explored and are discussed below.
Thermo-responsive Systems
Thermo-responsive materials undergo a phase change below or above a particular temperature. These changes are referred to as lower or upper critical solution temperature (LCST or UCST), respectively. Such materials are insoluble above or below the critical temperature but transform to a completely soluble form upon crossing the transition temperature. Poly(N-isopropyl acrylamide) (PNIPAAm) is one such polymeric material that is not ideal for drug delivery applications. PNIPAAm becomes hydrophobic at 32 °C in water and at temperatures that are similar to physiological conditions. However, altering the side chains, the molecular weight of the polymer, the polymeric architecture, or copolymerizing with other hydrophilic or hydrophobic polymers enables customization of the transition temperature to suit biomedical applications. PNIPAAm derivative, therefore, have been explored extensively as a material for thermo-sensitive drug delivery vectors and have been incorporated into micelles, liposomes, hydrogels and nanogels, polymersomes, interpenetrating networks, films, and the surface of inorganic nanoparticles.16 Copolymers of PNIPAAm with poly(N,Ndiethylacrylamide) (PDEAAm), poly(N-vinylcaprolactone) (PVCL), PLGA, poly[2-(dimethylamino) ethyl methacrylate] (PDMAEMA), PEG, gelatin, and chitosan have been used for the delivery of chemotherapeutic drugs and biologics. Beside drug delivery, considerable efforts have been made to develop thermo-sensitive materials for use in other applications including surfaces and scaffolds for tissue growth and engineering, imaging, and diagnostics.17
Light-responsive Systems
Light is a popular choice as an external stimulus since its intensity and penetration depth can be precisely controlled. As a result, light-sensitive materials have become increasingly popular as drug delivery systems. Azobenzene, o-nitrobenzene, coumarin, and pyrene derivatives are routinely used for devising light-responsive drug delivery vectors. Jiang et al. developed a UV-responsive micelle system with poly(ethylene oxide) (PEO) and poly(methacrylate) which included pyrene sidechains as diblock copolymers. The authors used Nile red dye (Prod. No. 19123) as a surrogate to demonstrate the efficient release of the payload as a function of irradiation time and illumination power.18 UV-irradiation, however, is not conducive for biological applications, especially for prolonged periods. For this reason, alternative materials responsive to visible or NIR wavelengths are being explored. Inorganic nanomaterials, especially anisotropic noble metal nanoparticles, show excellent NIR absorption characteristics and have been used as lightabsorbing materials to facilitate drug delivery. Kang et al. used silicacoated gold nanorods with strong absorption maxima around 850 nm as a template to polymerize crosslinked acrylamide on the surface of the nanoparticle. Doxorubicin (DOX) loaded particles showed enhanced cell cytotoxicity upon irradiation with NIR light (808 nm) but the nanoparticles that had not been irradiated or irradiation in the absence of nanoparticles showed little effect. These results confirmed light-mediated payload release.18 In a similar approach, Hribar, et al. developed a polymer-gold nanorod composite that allowed a controlled and precise release of small molecules (<800 Da) upon irradiation with NIR-light. The PbAE macromers (A6), tert-butyl acrylate (tBA) monomers, and 2-hydroxyethyl acrylate (10:20:70% w/w, respectively) were polymerized to form the polymer blend. A high concentration of gold nanorods and light-responsive material could then be loaded in the core of the polymer microparticles (Figure 3A–4C). In vitro release of loaded DOX demonstrated a strong dependence on the light irradiation (Laser ON) (Figure 3D) and generated increased toxicity in T6–17 cells due to light-induced drug release (Figure 3E).19
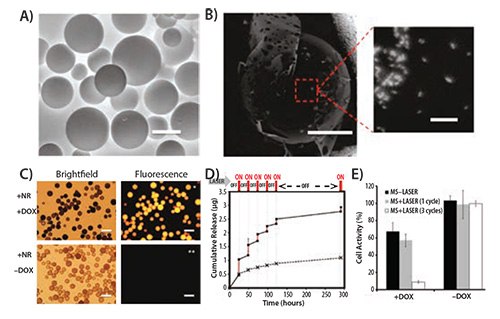
Figure 3.A) Environmental scanning electron micrograph of polymeric microspheres ( MS) made of A6:HEA:tBA (10:20:70). The scale bar corresponds to 50 μM. B) Backscattered micrograph of the microsphere showing the embedded gold nanorods (bright spots, scale bar = 20 μM) and a magnified image of the highlighted area showing the nanorods within the microsphere (scale bar = 200 nM). C) Bright field and florescence images of the microsphere showing the microspheres. The DOX loaded microspheres show intense fluorescence (top panel, right) while those without DOX do not show any background fluorescence (bottom panel, right). Scale bar in all images corresponds to 100 μM. D) Cumulative drug release from the microspheres as a function of laser pulse (Wavelength = 808 nm) applied at physiological temperature (37 °C). E) Histogram plot of T6–17 cells activity after exposure to MS alone with laser and DOX-loaded MS with a laser pulse of 1 and 3 cycles (Laser power = 1.1 W, 5 min/cycle). Reprinted with permission from Reference 19, American Chemical Society 2011.
Ultrasound-responsive Systems
The intensity of ultrasound energy such as light can be tailored for its intensity and focused over a small area in the body. This maximizes the drug release efficiency and, therefore, is often referred as high intensity focused ultrasound (HIFU). Due to its wide application in clinical imaging and diagnosis, ultrasound-based on-demand drug releasing vectors are seen as a natural material for the development of “theranostic” delivery systems. Ultrasound energy mediates drug release from a delivery vector by three main mechanisms: (1) heat generation, (2) acoustic cavitation, and (3) acoustic radiation forces. Some of the important parameters which can influence the performance of ultrasound-based techniques include the time and nature of application. The rationale behind ultrasound-based delivery is largely regulated by heat generation; therefore, the nanoparticle delivery systems designed for ultrasoundbased delivery are similar in composition to thermo-responsive systems. Microbubble technology, initially developed for contrast enhancement in ultrasound imaging, has since been exploited for acoustic cavitation to alter cell permeability and has also been used as a delivery vehicle.20
Ibsen, et al. prepared nested liposomes synthesized by a method in which a microbubble was created within the liposome to impart ultrasoundresponsiveness and demonstrated that it could be used for delivery of both small and large molecules. Similarly, mRNA-lipoplex loaded microbubbles were employed as a vaccine to successfully transfect and express the reporter gene in dendritic cells. This led to a slight shift in maturation and, in turn, induced T-cell response.21 As a result, ultrasonicassisted delivery is an attractive approach with significant potential.
Magnetically Regulated Systems
Like thermo- and ultrasound-responsive vectors, delivery systems responsive to magnetic fields rely on the induction of hyperthermia to release the payload. The ability to direct and concentrate these vectors in a specific area of the body through the application of an external magnetic field gives an added advantage to such delivery systems. Traditionally, magnetic nanoparticles such as superparamagnetic iron oxide nanoparticles (SPIONs) are incorporated into polymeric, lipidic, or protein delivery systems to impart them with magnetic properties. SPIONs have also been extensively used as magnetic resonance imaging (MRI) contrast agents, and their presence in a delivery vector offers an imaging modality that possesses stimuli-responsiveness. SPIONs coated with the thermo-responsive polymer PNIPAAm and loaded with DOX showed a rapid drug release above the lower critical solution temperature (LCST) due to magnetic field induced hyperthermia but a slow sustained release below the LCST. In vivo studies in buffalo rats implanted with hepatocellular carcinoma revealed magnetically guided increased accumulation and drug release in tumors. This resulted in an improved contrast-based MR imaging and efficient therapeutic potential.22 Majewski, et al. demonstrated the utility of the magnetically active vectors as a gene delivery system. Most importantly, the study demonstrated that internalized magnetic nanoparticles can be used for selective isolation of the transfected cells from the population.23 Due to the added versatility of the magnetically regulated delivery system, they are now often used to design dual and multiple stimuli-responsive vectors to harvest the benefits of individual stimuli-responsiveness.24
Electro-responsive Systems
Certain materials (organic and inorganic) exhibit conductive properties and can be used to design delivery systems that are responsive to an external electric field. Common examples of electro-sensitive materials for drug delivery include polypyrrole (PPy, Prod. No. 577030), ferrocene, and carbon nanotubes. Weak electric pulses (~1 V) are generally used for such applications and these electric fields are preferred over other externally applied stimuli due to several advantages: an electric pulse is (1) easy to control and apply, (2) does not need sophisticated and elaborate instrumentation, and (3) can be easily integrated to design chip-based devices. Ge, et al. prepared dodecyltrimethylammonium bromide (DTAB) micelles with decyl alcohol as a cosurfactant, and PPy was polymerized in the hydrophobic core. These nanoparticles were then loaded with a thermo-sensitive PLGA-PEG-PLGA block polymer that showed temperature-dependent sol-gel transformation. The polymer exists as a solution at 4 °C but rapidly forms a gel at a physiological temperature of 37 °C. Daunorubicin and fluorescein were loaded into the nanoparticles that were embedded in the polymer matrix. The resulting material maintained its solid hydrogel form at body temperature and demonstrated an electric-pulse dependent drug release. The subcutaneously injected, soluble form rapidly forms a gel in FVB mice and successfully released the payload in vivo upon application of the electric pulse. In the control group, the absence of an external stimulus resulted in insignificant release of the cargo.25 Adopting a novel approach, Zhu, et al. grafted 4-(3-cyanophenyl) butylene (CPB) as an electric field-active “nanoimpeller” onto the wall of mesoporous silica. Due to their large inherent dipole moment, the grafted CPBs reorient under the influence of an applied electric field. The CPBs then rapidly release the guest molecules (ibuprofen) from their pores.26
Future Perspectives
The plethora of available literature on stimuli-responsive delivery systems demonstrates the growing importance for these systems. However, a majority of these systems have not made it past the pre-clinical stage and only a handful of examples currently have entered clinical trials.27 The need for a precise control over the “response” to the applied “stimulus” makes their clinical translation challenging. The complex synthetic steps and formulation of multiple components further compounds the issue. The majority of stimuli-responsive delivery systems are still in the early stages of development and the optimization of the synthesis procedures is needed before they can transition into the clinical world. Accuracy and precision over the stimulus will also need improvement from preclinical to the clinical level. Externally applied “physical” stimuli are easy to control and manipulate but internal “biological” triggers are not as easily controlled. Tumors show considerable variation in their physiological status between patients, organs, or even the same tumors in different species. External stimuli, on the other hand, need improvement to achieve better tissue penetration without causing any damage, which would require the optimization of several contributing parameters.
Other factors can also have a negative impact on delivery systems for the stimuli-responsive vectors. The EPR effect that leads to accumulation of the delivery vectors into the tumor is a well-accepted phenomenon in preclinical studies but has not yet been confirmed in clinical settings. Additionally, a majority of diseases show a complex microenvironment consisting of diseased cells, tissue interstitium, immune cells and other structural cells of the tissue that limits the ability of the delivery system to access the desired target cells. These physical and physiological barriers further impede the optimal performance of these delivery systems. In the case of cancer, heterogeneity at the cellular and physiological levels significantly limits the ability of these vectors to access their targets. A majority of the stimuli-responsive systems discussed have been tested in vitro but few have in vivo applications, an aspect that needs immediate focus. The goal is to design simplified systems with positive stimuliresponsive characteristics. This achievement will drastically improve the chances for clinical applications. Though several major hurdles are yet to be overcome, stimuli-responsive systems have ultimately shown tremendous promise as alternatives to the existing delivery approaches.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?