Strat-M® Transdermal Diffusion Membrane Performance
Compatibility Data
Strat-M® membrane has broad chemical compatibility and is an appropriate test model for compounds across a wide range of physiochemical properties. Seven test compounds ranging in molecular weight from 180 to 392 with log P values (representing relative lipophilicity) of -0.131 to 6.9 were tested for diffusion through human skin and Strat-M® membrane. The flux of each compound through human skin and through Strat-M® membrane was nearly equivalent, with a mean flux ratio of 1.14.

Figure 1.
Data points were calculated from the ratios of average values for cumulative flux of seven compounds through Strat-M® and human cadaver skin, measured at 8 hours. The solid line represents the ideal 1:1 correlation. More details on the test conditions can be found on the flux charts of the individual compounds.
Diffusion Rate
Whether you are safety testing a slowly diffusing sunscreen active, or optimizing a rapidly diffusing NSAID formulation for pain relief, Strat-M® membrane provides the versatility to generate meaningful data.
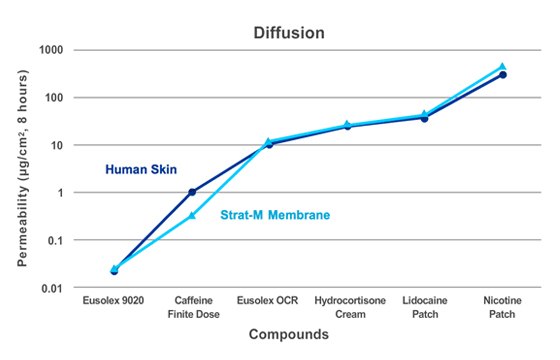
Figure 2.
Transdermal diffusion testing was conducted for several formulations with varying diffusion rates to assess correlation between Strat-M® membrane and human cadaver skin. Six Franz cells were used for each formulation—three with Strat-M® membrane and three with human cadaver skin. The donor chambers were loaded with 500 µL of the formulations. Samples were collected hourly and analyzed by HPLC. The results showed correlation between human skin and Strat-M® membrane over a range of diffusion rates.
Sensitive to Enhancers
Strat-M® membrane exhibits differential permeability in the presence of enhancers, so it is appropriate for use during formulation optimization.
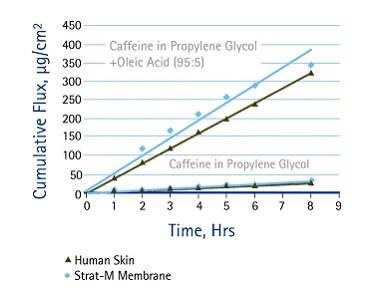
Figure 3.
Caffeine Solution in Propylene Glycol & Propylene Glycol + Oleic Acid showing similar diffusion behavior between human skin and Strat-M® membrane as a permeation enhancer (Oleic acid) is added to the formulation.
Variability Data
No need to repeat testing each time a new lot of skin arrives in the lab. Strat-M® membrane is a highly consistent, engineered material that allows you to compare today’s diffusion data with data you will generate next week, or next month.
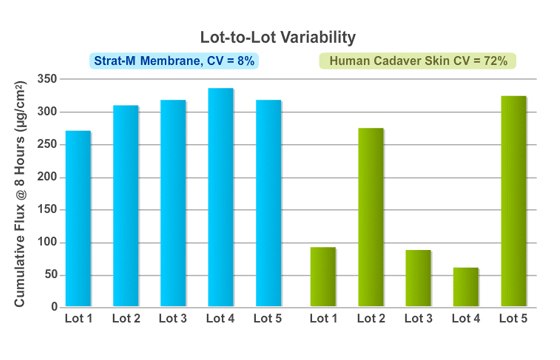
Figure 4.
Measured flux rate of a caffeine solution through Strat-M® membrane (left) shows greater lot-to-lot consistency than through human cadaver skin (right). Flux rates of caffeine were measured for five different lots of Strat-M® membrane and eight different lots of human cadaver skin. The diffusion test was conducted in a Franz cell with a 500 µL saturated caffeine solution. Data represent average 8-hour cumulative flux values for four samples per lot of membrane or skin. The average flux for Strat-M® membrane was 304.0 µg/cm2, with CV = 8%. The average flux for human cadaver skin was 168.8 µg/cm2, with CV = 72%. Strat-M® membrane used in this study was pre-validated material.
Compound Data
Strat-M® Membrane Can Predict Transdermal Diffusion of a Variety of Compounds.
Strat-M® membrane is predictive of diffusion of human skin for a wide variety of active compounds and formulations. Use the Compound Correlation Tool to learn how Strat-M® membrane will work for you.
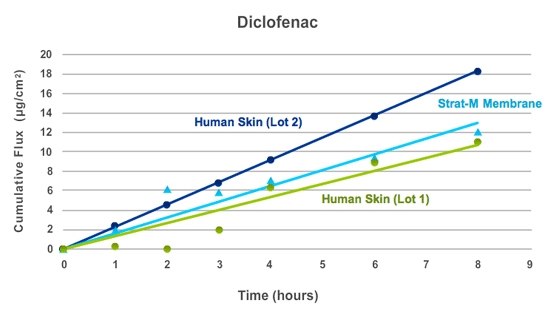
Figure 5.
Strat-M® membrane was tested alongside two lots of human cadaver skin in a Franz diffusion cell apparatus (0.635 cm2). Three Franz cells were used for Strat-M® membrane and each lot of skin. The donor compartment of each cell was loaded with 500 µL of a saturated diclofenac solution. Data represents average cumulative flux at each test point.
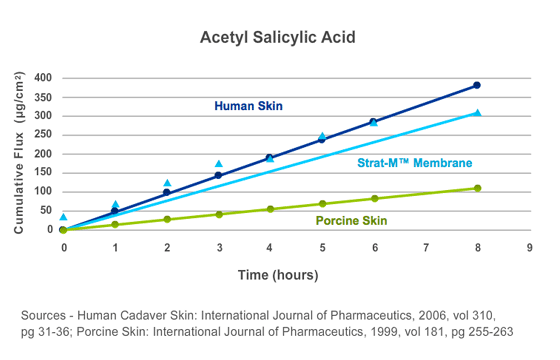
Figure 6.
The diffusion rate of acetyl salicylic acid (Aspirin) through Strat-M® membrane was measured on a Franz diffusion cell
apparatus (0.635 cm2). Six Franz cells were used. The donor compartment of each cell was loaded with 500 µL of a saturated acetyl salicylic acid solution. Diffusion performance was compared to published values for 8-hour cumulative diffusion rate through human cadaver and porcine skin. Data represents average cumulative flux at each test point.
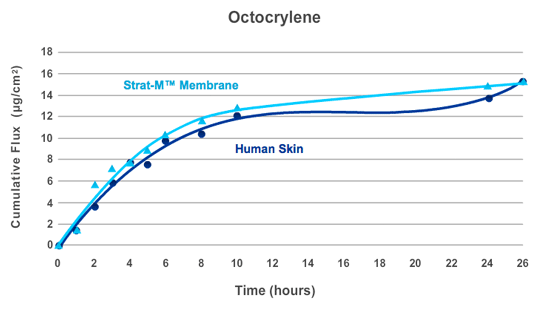
Figure 7.
Strat-M® membrane was tested alongside human cadaver skin in a Franz diffusion cell apparatus (0.635 cm2). Three Franz cells were used for each test model. The donor compartment of each cell was loaded with 500 μL of a Octocrylene emulsion (3.5%). Data represents average cumulative flux at each test point.
Note: Information presented is based on preliminary development tests. Official claims will be based on product validation results.
To continue reading please sign in or create an account.
Don't Have An Account?