Selectophore™ Ionophores
Highly pure and stable compounds for the preparation of ion
Introduction
Chemical sensors are devices that can quantitatively and reversibly measure a particular analyte. Due to their specificity and sensitivity, they are becoming more and more prevalent in chemical analysis, environmental monitoring, medicine and food analysis, among other areas. Many chemical sensors have been developed and commercialized in the past few decades, with more under development to keep pace with new analytical challenges.
Chemical sensors are often used in an electrode format which is then immersed in the sample to be analyzed. Presence of the target analyte is evidenced by a change in the electrode potential. The principle of the membrane preparation for ion-selective electrodes (ISE) is to incorporate an organic compound as the ionophore into a polymer (e.g. polyvinyl chloride) membrane together with an appropriate plasticizer and additive which provide the membrane with the properties of a liquid phase (Figure 1). This approach can also be used to prepare optical sensor membranes.
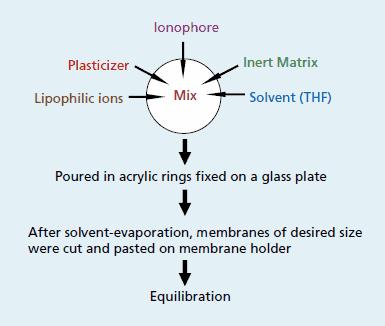
Figure 1Preparation of an ionselective membrane for potentiometric sensors
Selectophore™ Grade Ionophores
Selectophore™ from Sigma-Aldrich is the most comprehensive product line available to fulfill requirements for the preparation of sensor membranes for ion-selective potentiometric and optical devices. Ionophores and auxiliary components (polymers, plasticizers, additives) used in potentiometric or optical sensors have different specifications than materials used for other applications. It is essential that the product is free of disrupting impurities, such as metal ions or surfactants. However, the specific purity requirements are a function of the analyte, with some analyteionophore combinations more sensitive to impurities than others. For this reason, all Selectophore™ ionophores are application tested.
For a complete list of our Selectophore™ product range, please visit the web site: www.sigmaaldrich.com/ sensoric.
Note: Patents for synthesis or applications may apply.
Selectophore‚ Calcium Ionophore I (ETH 1001)
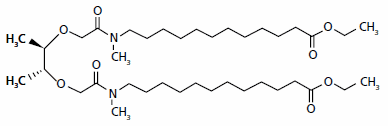
ETH 1001, CAS# 58801-34-6
Function-tested, ≥ 99.0% (HPLC)
Cat. No. 21192
Calcium Ionophore I, also known as ETH 1001, is a neutral ionophore with extremely high selectivity for Ca2+ ions. The synthesis and purification of Calcium Ionophore I requires a great deal of expertise. Over the last two decades, Sigma-Aldrich researchers have improved the synthesis and purification of this compound and can now offer Calcium Ionophore I with purity and performance unmatched in the market. The recent synthesis optimization resulted in a highly stable product with shelf life exceeding four months.
Characteristics:
Linear range: 2 x 10-7 to 1 x 10-1 mol/L (CaCl2)
Slope (sensitivity): 28 mV/dec
Detection limit: 1 x 10-7 mol/L
Shelf life: >4 months
Selectophore‚ Ammonium Ionophore I (nonactin)
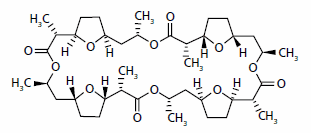
Nonactin, CAS# 6833-84-7
Function-tested
Cat. No. 09877
Ammonium Ionophore I is an antibiotic isolated from fermentation. Although this ionophore is very selective for NH4+ ions, it can also be used for the determination of urea after enzymatic decomposition.
Characteristics:
Linear range: 1 x 10-6 to 1 x 10-1 mol/L (NH4Cl)
Slope (sensitivity): 60.8 mV/dec
Detection limit: 6 x 10-7 mol/L
Shelf life: >4 months
The example in Figure 2 illustrates the advantage of the Selectophore brand over other quality grades. Although Ammonium Ionophore I is simply the compound nonactin, there are enormous differences in the quality derived from various sources. Competitive products often contain ionic impurities, which leach out over a period of time causing anomalous baselines.
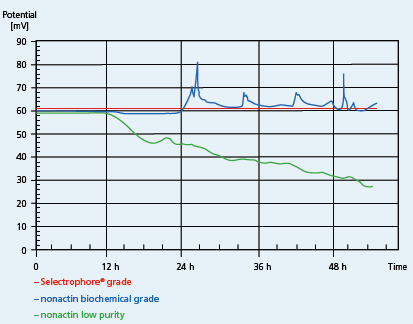
Figure 2Comparison of different quality grades of nonactin. Source: Nadler Technologies, Switzerland (www.nadler.ch)
Materials
To continue reading please sign in or create an account.
Don't Have An Account?