Immunohistochemistry Procedure for Paraffin-Embedded Tissues
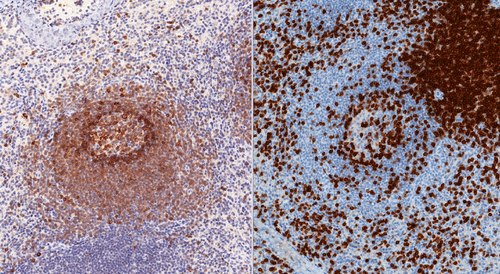
Figure 1.Immunohistochemical staining
Immunohistochemistry Technique
Immunohistochemical staining is a valuable tool for detecting specific antigens in tissues. In order to perform the standard staining procedure, first the tissue section has to be deparaffinized and then rehydrated before applying the primary antibody. Enzyme-conjugated secondary antibodies are then applied and the specific staining can be visualized after adding the enzyme-specific substrate. Occasionally, when weak or no staining is observed, an antigen "unmasking" by enzyme digestion, may be required.
The following procedure describes the application of peroxidase or alkaline phosphatase conjugates in the immunohistochemical labeling of formalin-fixed, paraffin-embedded tissue sections.
Reagents and Equipment
Find many of these products in the materials table below.
- Formalin-fixed, paraffin-embedded tissue sections
- 0.01 M phosphate buffered saline, pH 7.4 (PBS) (Product No. P3813 or P4417-tablet)
- Bovine serum albumin (BSA) (Product No. A9647)
- Diluent: 1% BSA in PBS (Product No. P3688)
- Xylene (Product No. 247642, or Product No. 534056)
- Ethanol absolute (Product No. 32221, Europe only, or Product No. E7023)
- 0.1% Trypsin (Product No. T7409) in PBS or Trypsin tablet (Product No. T7168) in 4mM CaCl2, 200 mm Tris, pH 7.7, or 0.1% Protease (Product No. P5380) in PBS.
- Microwave antigen retrieval solution: 10 mM sodium citrate (Product No. C8532) buffer pH 6.0, 1 mM EDTA (Product No. ED4SS) pH 8.0
- Primary antibody
- Option 1: Biotinylated secondary antibody and ExtrAvidin-Peroxidase (Product No.
E2886) or ExtrAvidin-Alkaline Phosphatase (E2636), or Option 2: Secondary antibody conjugated to peroxidase or alkaline phosphatase - When using peroxidase-conjugated secondary antibodies or ExtrAvidin-Peroxidase: 3% hydrogen peroxide (in deionized water, freshly prepared from 30% stock).
- Enzyme substrate: depending on the enzyme and color needed (see Substrates).
- For peroxidase: AEC Staining Kit (Product No. AEC101), DAB (Product No. D4418 or D0426).
- For alkaline phosphatase: Fast Red TR/Naphtol AS-MX (Product No. B5655).
- Mayer's hematoxylin (Product No. MHS1)
- Coplin staining jars (Product No. S5641)
- Microwave
- Microscope
IHC Protocol Steps
- Slide Preparation
- Primary Antibody Reaction
- Secondary Antibody Reaction
- Substrate Preparation
- Development
- Counterstaining
Slide Preparation
- Deparaffinization and Rehydration
- Antigen Retrieval - Unmasking
- Inactivation of Endogenous Peroxidase
Deparaffinization and Rehydration
- Place the slides in a 56-60 °C oven for 15 min. (Caution: Oven temperature must not exceed 60 °C).
- Transfer to a xylene bath and perform two changes of xylene for 5 min. each.
- Shake off excess liquid and rehydrate slides in two changes of fresh absolute ethanol for 3 min. each.
- Shake off excess liquid and place slides in fresh 90% ethanol for 3 min.
- Shake off excess liquid and place slides in fresh 80% ethanol for 3 min.
- Rinse the slides in gently running tap water for 30 seconds (avoid a direct jet which may wash off or loosen the section).
- Place in PBS wash bath for further rehydration (30 min. at room temperature).
Antigen Retrieval - Unmasking of Antigen
Note: This step is performed only in cases where weak or no staining occurs, or for antigens requiring "unmasking" according to the primary antibody specifications.
There are several possible ways for retrieval depending on the antibody and the antigen:
Enzyme retrieval:
- Apply 0.1% trypsin in PBS or 0.1% protease in PBS for 2-30 min. at 37 °C. Extending the incubation time may also enhance specific staining. Rinse in PBS for 10 min.
Microwave retrieval:
- Wash the slides with deionized H2O and place them in a microwave-resistant plastic staining jar containing antigen retrieval solution. Make sure slides are fully covered with solution.
- Operate the microwave oven for 5 min. on high power (~700 watts). Make sure slides are still covered with retrieval solution or add fresh solution and repeat microwaving.
- This process can be repeated 2-3 times.
- Let cool slowly at room temperature for at least 20 min. before proceeding to the next step.
Inactivation of Endogenous Peroxidase
Note: This step is performed only when using peroxidase-conjugated secondary antibodies or ExtrAvidin-Peroxidase.
- Place the slides on a flat level surface. Do not allow slides to touch each other. Do not allow the sections to dry out at any time.
- Add enough drops of 3% hydrogen peroxide to cover the whole section.
- Incubate 5 min. at room temperature.
- Rinse with PBS from a wash bottle.
- Place the slide in PBS wash bath for 2 min.
Primary Antibody Reaction
Note:
a. Pre-incubation of the sample with 5% BSA for 10 min. prior to the primary antibody reaction may decrease background staining. For best results with animal tissues, use 5 to 10% normal serum from the same species as the host of the secondary antibody.
b. Optimal dilution and incubation times should be determined for each primary antibody prior to use.
- Allow the slides to drain, shake off excess fluid with a brisk motion and carefully wipe each slide around the sections.
- Dilute the primary antibody or negative control reagent to its optimal dilution in diluent. The diluent alone may be used as a negative control. A positive control slide (a tissue known to contain the antigen under study) should also be run.
- Apply 100 µL primary antibody solution to the appropriate slides, covering the tissue sections.
- Tilt each slide in two different directions, so the liquid is spread evenly over the slide.
- Incubate for at least 60 min. at 37 °C in humidified chamber. Longer incubations are advised for low density antigens.
- Rinse gently with PBS from a wash bottle. Place the slide in a PBS wash bath for 5 min.
Secondary Antibody Reaction
Option 1 - Biotin/ExtrAvidin Detection
- Allow the slides to drain, shake off excess fluid and carefully wipe the slide as before.
- Dilute the biotinylated secondary antibody in diluent to its optimal concentration.
- Apply 100 µL to each slide, covering the tissue sections.
- Tilt each slide in two different directions.
- Incubate in a humidity chamber for at least 30 min. at room temperature.
- Rinse gently with PBS from a wash bottle.
- Place the slide in a PBS wash bath for 5 min.
ExtrAvidin Reaction
- Allow the slides to drain. Shake off excess fluid and carefully wipe the slide as before.
- Dilute ExtrAvidin peroxidase or ExtrAvidin alkaline phosphatase, in diluent to its optimal concentration.
- Apply 100 µL to all slides; cover the section.
- Tilt each slide in two different directions.
- Incubate in humidified chamber for at least 20 min. at room temperature.
- Rinse gently with PBS from a wash bottle.
- Place the slide in PBS wash bath for 5 min.
- Skip to Substrate Preparation and Development step.
Option 2 - Enzyme-labeled Secondary Antibody
- Allow the slide to drain. Shake off excess fluid with a brisk motion and carefully wipe the slide as before.
- Dilute the peroxidase or alkaline phosphatase conjugated secondary antibody in the diluent to its optimal dilution.
- Apply 100 µL to all slides, covering the tissue sections.
- Tilt each slide in two different directions.
- Incubate 30 min. at room temperature or at 37 °C in humidified chamber.
- Rinse gently with PBS from a wash bottle.
- Place the slides in a PBS wash bath for 5 min.
Substrate Preparation
It is recommended to prepare the substrate mixture during the final wash step.
Note: Addition of 1 mM levamisole (Product No. L9756) to the alkaline phosphatase substrate solution will largely inhibit endogenous alkaline phosphatase activity (except that of the intestinal isoenzyme)
Development
- Allow each slides to drain. Shake off excess fluid and carefully wipe the slide as before.
- Apply enough drops of freshly prepared substrate mixture to cover the tissue section.
- Incubate 5-10 min. or until desired color reaction is observed when monitored with the microscope. Terminate the reaction before background staining appears in the negative controls by rinsing gently with distilled water from a wash bottle.
Counterstaining
Note: When using AEC substrate, do not use alcohol-containing solutions for counter-staining (e.g., Harris' hematoxylin, acid alcohol), since the AEC stain formed by this method is soluble in organic solvents. The slide must not be dehydrated, brought back to toluene (or xylene), or mounted in toluene-containing mountants.
- Apply enough Mayer's hematoxylin to cover the section or place the slide in a bath of Mayer's hematoxylin.
- Incubate for 0.5-5 min., depending on strength of the hematoxylin used.
- Rinse the slide gently with distilled water from a wash bottle.
- Rinse the slide under gently running tap water for 5 min. (avoid a direct jet which may wash off or loosen the section).
- Mount the sections using aqueous mounting medium such as glycerol gelatin. Coverslip may be sealed with clear nail polish.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?