Trilineage Differentiation of Multipotent Human Mesenchymal Stem Cells (MSCs) into Osteocytes, Adipocytes and Chondrocytes
Mesenchymal stem cells (MSCs) are fibroblastoid multipotent adult stem cells with a high capacity for self-renewal and differentiation. These cells have been isolated from several human tissues, including bone marrow, adipose tissue, umbilical cord matrix, tendon, lung, and the periosteum.1 Recently it has been shown that MSCs originate from the perivascular niche, a tight network present throughout the vasculature of the whole body. These perivascular cells lack endothelial and hematopoietic markers, e.g. CD31, CD34, and CD45, but express CD146, PDGFR beta, and alkaline phosphatase.2 According to the position paper published by the International Society for Cellular Therapy (ISCT), MSC express the surface markers CD73, CD90, and CD105 and stain negative for CD14 or CD11b, CD34, CD45, CD79α or CD19, and HLADR.3 In addition to surface marker analysis, the most common and reliable way to identify a population of MSC is to verify their multipotency. MSC can differentiate into adipocytes, osteoblasts, myocytes, and chondrocytes in vivo and in vitro.1,4 Transdifferentiation of MSC into cells of nonmesenchymal origin, such as hepatocytes, neurons, and pancreatic islet cells, has also been observed in vitro when specific culture conditions and stimuli are applied.1 The directed differentiation of MSCs is carried out in vitro using appropriate differentiation media, such as the ready-to-use PromoCell MSC Differentiation Media (C-28016, C-28012, C-28014, C-28013, C-28015).
Read more about
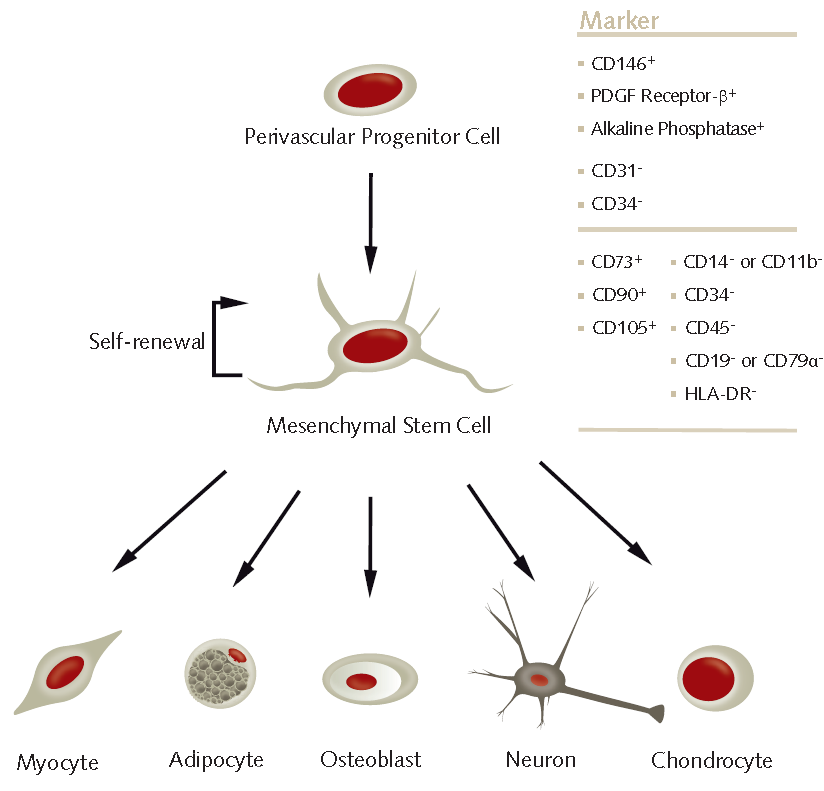
Figure 1. In vitro differentiation of human MSCs.Under appropriate culture conditions, multipotent human mesenchymal stem cells can differentiate into osteoblasts, adipocytes, chondrocytes, myocytes and neurons.
Osteogenesis Differentiation Protocol
- Coat the culture vessel. Coat a 6-well tissue culture plate with 10 μg/mL human fibronectin or bovine fibronectin according to the instruction manual.
- Seed Mesenchymal Stem Cells. Plate MSCs at 1x105 cells per well in the fibronectin-coated tissue culture plate using MSC Growth Medium 2 (C-28009). Work in duplicate.
- Allow Mesenchymal Stem Cells to grow. Allow the cells to reach 80-90% confluency. This will take 24-48 hours.
- Induce Mesenchymal Stem Cells. Induce one of the duplicate samples with MSC Osteogenic Differentiation Medium (C-28013). Use MSC Growth Medium 2 for the remaining well as a negative control.
- Induce Mesenchymal Stem Cells. Incubate for 12-14 days. Change the medium every third day. Be careful not to disturb the cell monolayer.
- Wash the cells. After differentiation steps are complete, remove the cells from the incubator and carefully aspirate the medium. Gently wash the cells with Dulbecco’s phosphate buffer saline (PBS) w/o Ca++/Mg++ (D8537).
- Fix the cells. Carefully aspirate the PBS and add enough Saccomanno Fixation Solution to cover the cellular monolayer. After at least 30 min gently aspirate the fixation solution and wash the cells with distilled water.
- Stain the cells. Immediately before use, pass the required amount of Alizarin Red S staining solution (TMS008) through a 0.22 μm Millex PES filter. Carefully aspirate the distilled water and add enough filtered Alizarin Red S staining solution to cover the cellular monolayer. Incubate at room temperature in the dark for 10-15 min. Monitor staining progress for 10 min and stop the process when staining intensity is sufficient.
- Wash the cells. Carefully aspirate the Alizarin Red S staining solution and wash the cell monolayer four times with 1 mL distilled water. Carefully aspirate the distilled water and add PBS.
- Analyze the cells Analyze the sample immediately, as the dye may bleed upon prolonged storage without embedding. Undifferentiated MSC (without extracellular calcium deposits) are colorless/slightly purple, whereas MSC-derived osteoblasts (with extracellular calcium deposits) stain bright orange-red (Figure 1).
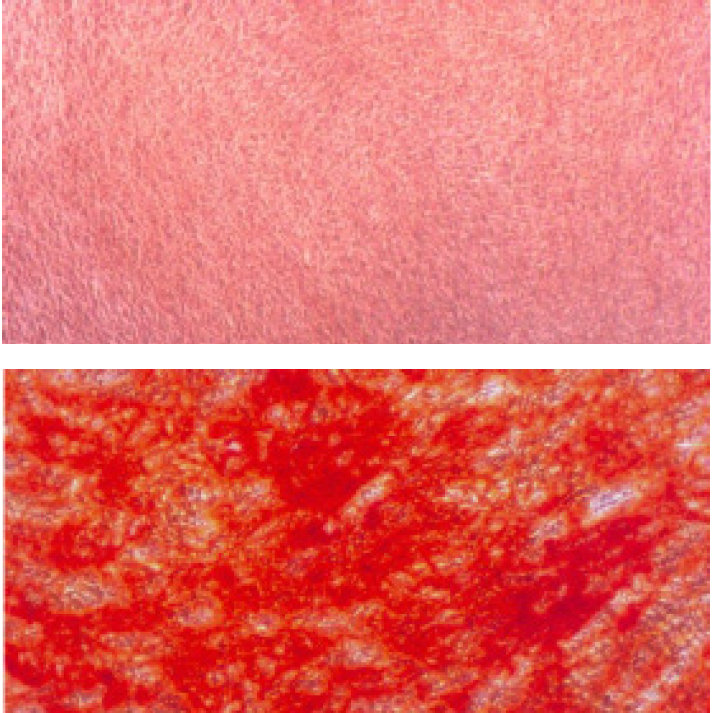
Figure 2.Alizarin Red S staining of extracellular calcium deposits in mineralized hMSCBM derived mature osteoblasts. MSC cells were cultured for 12 days in PromoCell MSC Growth Medium 2 for the negative control (upper panel) or MSC Osteogenic Differentiation Medium for the differentiation sample (lower panel). In contrast with the negative control, the mature osteoblasts differentiated from MSC show intense redorange staining of mineralized bone matrix. Note also the concentration of Alizarin Red S staining in some of the larger bone nodules.
Adipogenesis Differentiation Protocol
- Coat the culture vessel. Coat a 6-well tissue culture plate with 10 μg/mL human fibronectin or bovine fibronectin according to the instruction manual.
- Seed the Mesenchymal Stem Cells. In a fibronectin-coated 6-well tissue culture plate, plate 1 x 105 MSC per well using MSC Growth Medium 2 (C-28009). Work in duplicate.
- Allow the Mesenchymal Stem Cells to grow. Allow the cells to reach 80-90% confluency. This will take 24-48 hours.
- Induce the Mesenchymal Stem Cells. Induce one of the duplicate samples with MSC Adipogenic Differentiation Medium 2 (C-28016). Use MSC Growth Medium 2 for the remaining well as a negative control.
- Differentiation of the induced Mesenchymal Stem Cells. Incubate for 12-14 days. Change the medium every third day taking care not to disturb the cell monolayer.
- Wash the cells. After differentiation steps are complete, remove the cells from the incubator and carefully aspirate the medium. Gently wash the cells with Dulbecco’s phosphate buffer saline (PBS) w/o Ca++/Mg++ (D8537).
- Fix the cells. Carefully aspirate the PBS (D8537) and add enough Saccomanno Fixation Solution to cover the cell monolayer. Incubate at room temperature for at least 30 min.
- Wash the cells. Carefully aspirate the fixation buffer and wash the cell monolayer with distilled water. Gently aspirate the water and add enough 60% isopropanol to cover the cell monolayer. Incubate at room temperature for 5 min.
- Stain the cells. Carefully aspirate the 60% isopropanol and add enough OilRedO staining solution (O1391) to cover the cell monolayer. Incubate at room temperature for 10-15 min.
- Wash the cells. Carefully aspirate the staining solution and wash the cell monolayer several times with distilled water until the water is clear. Blot the vessel containing the stained cells upside down on a paper towel to remove as much water as possible.
- Analyze the cells. Cover with PBS and analyze the stained samples promptly as the dye tends to fade upon prolonged light exposure. Intracellular lipid vesicles in mature adipocytes will be stained bright red.
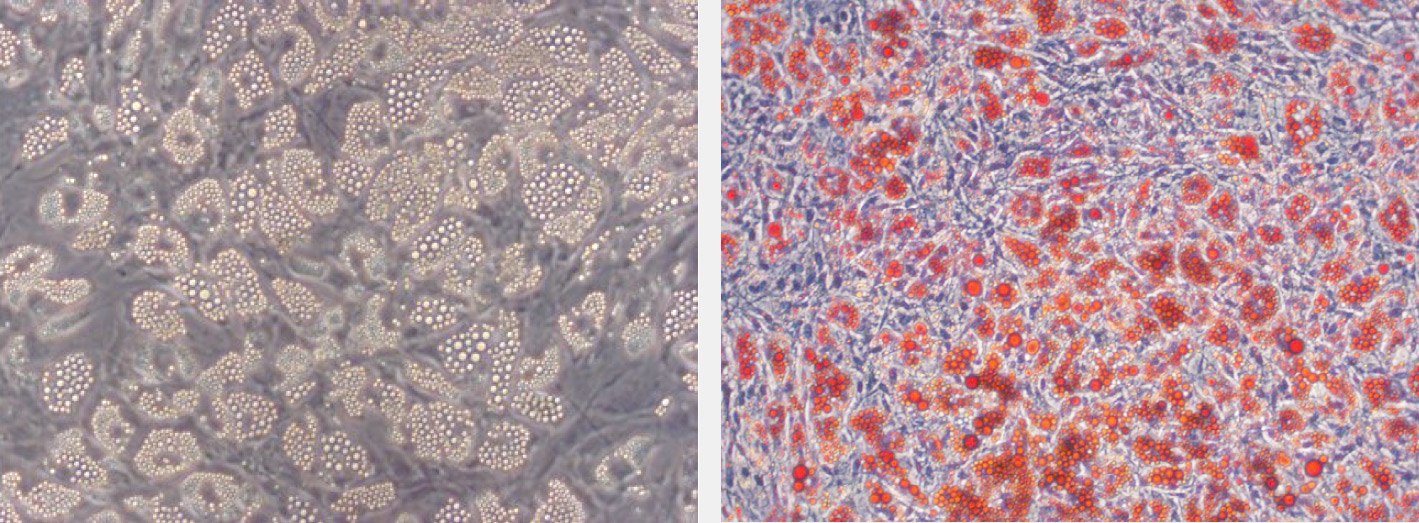
Figure 3. Oil Red O staining of lipid droplets in hMSC-BM derived adipocytes.Lipid vesicle accumulation in adipocytes differentiated from hMSC-BM (human MSC derived from bone marrow) using the PromoCell MSC Adipogenic Differentiation Medium 2. The differentiated culture exhibits extensive intracellular lipid vacuole formation typical of mature adipocytes (left: 40x magnification; right: 100x magnification).
Chondrogenesis Differentiation Protocol
- Preparation of the Negative Control Medium. The negative control medium is Dulbecco’s Modified Eagle’s Medium (DMEM, low glucose) with 2 mM L-glutamine and 10% fetal bovine serum.
- Seed Mesenchymal Stem Cells. Plate MSC at 2 x 105 cells per well in a 96-well Ubottom suspension culture plate using the negative control medium. Work in duplicate.
- MSC-spheroid formation. Spheroids will spontaneously form within 24-48 hours of incubation.
- Induce MSC-spheroids. Induce one of the duplicate samples with MSC Chondrogenic Differentiation Medium (C-28012). Use the negative control medium for the remaining wells.
- Differentiate induced MSC-spheroids. Incubate for 21 days. Change the medium every third day taking care not to aspirate the spheroids.
- Wash the cartilage spheroids. After differentiation steps are complete, remove the cells from the incubator and carefully aspirate the medium. Gently wash the spheroids with Dulbecco’s phosphate buffer saline (PBS) w/o Ca++/Mg++ (D8537).
- Fixation of the cartilage spheroids. Carefully aspirate the PBS. Add enough Saccomanno Fixation Solution to cover the cartilage spheroids. Incubate at room temperature for 3 hours.
- Wash the cartilage spheroids. Carefully aspirate the fixative and wash the spheroids twice with distilled water.
- Stain the cells. Immediately before use, pass the required amount of Alcian Blue staining solution (TMS010) through a 0.22 μm Millex PES filter. Carefully aspirate the distilled water and add enough filtered Alcian Blue staining solution to generously cover the cartilage spheroids, as some evaporation will occur. Incubate in the dark for 45 minutes at room temperature.
- Wash the cells. Carefully aspirate the Alcian Blue staining solution and wash the cartilage spheroids with the destaining solution for 10 min. Repeat the wash step twice. Carefully aspirate the destaining solution and add PBS.
- Analyze the cartilage spheroids. Cartilage will be stained an intense dark blue, whereas other tissue will, at most, stain light blue.
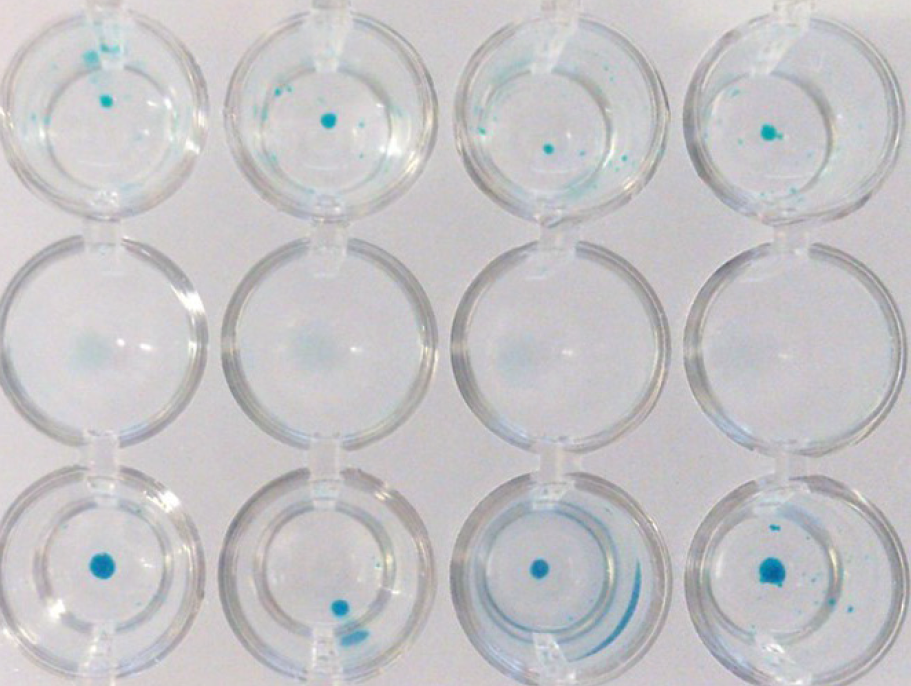
Figure 4. Alcian Blue staining of hMSC-BM derived chondrocytes.MSC spheroids after in vitro differentiation into cartilage using PromoCell MSC Chondrogenic Differentiation Medium. Spheroids cultured in the negative control medium stain light blue (upper row). In contrast, the induced spheroids exhibit an intensely blue color indicative of cartilage extracellular matrix (lower row).
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?