GC Analyses of FAMEs by Boiling Point Elution
Introduction
For the food chemist, determining the fatty acid composition of a product may be diffi cult because foods can contain a complex mixture of saturated, monounsaturated, and polyunsaturated fatty acids, each with a variety of carbon chain lengths. Many specialised products, such as GC columns and chemical standards, exist specifi cally for use in the quantitative identifi cation of fatty acids. Each of these products is manufactured with the chromatographer in mind, to help them ensure accurate and reproducible analyses.
Read more about
FAMEs by Boiling Point Elution
GC can be used to analyse fatty acids either as free fatty acids or as fatty acid methyl esters. There are distinct reasons for either choice. Short chain, more volatile fatty acids are typically analysed in the free form using specialised columns. The main benefi ts are the ease/ speed of sample preparation and the lack of artefacts in the analysis resulting from a derivatisation procedure. Fatty acids can also be analysed as fatty acid methyl esters. The main reasons include:
- In their free, underivatised form, fatty acids may be difficult to analyse because these highly polar compounds tend to form hydrogen bonds, leading to adsorption issues. Reducing their polarity may make them more amenable for analysis.
- To distinguish between the very slight differences exhibited by unsaturated fatty acids, the polar carboxyl functional groups must first be neutralised. This then allows column chemistry to perform separations by boiling point elution, and also degree, position, and even the cis vs. trans configuration of unsaturation.
The previous article in this Reporter covers the analysis of free fatty acids1. Previous Reporter articles have detailed the GC analysis of omega 3 and omega 6 fatty acids as FAMEs and cis/trans fatty acid isomers as FAMEs2-3. This article will focus on the analysis of FAMEs by boiling point elution, used for pattern recognition. This technique is useful for:
- Determining the source of fatty acids when compared to patterns/ profiles from known references, each with a unique fatty acid distribution. Qualitative and quantitative analysis is fundamental to food manufacturers for quality control, purity determination, and for the detection of adulterants.
- Observing subtle differences from sample to sample, allowing the effects on fatty acid metabolism, caused by either external or internal influences, to be detected. This growing area of research is commonly referred to as metabolomics, and extends to compound classes beyond fatty acids.
GC Column Choices
The separation of analytes in a boiling point elution requires the use of a non-polar GC column. The Equity-1, a rugged non-polar column, can be used for this application with great success. Column specifi - cations for the Equity-1 are shown in Table 1.
A chromatogram of the Supelco 37-Component FAME Mix on the Equity-1 is presented in Figure 1. As shown, this column possesses the necessary column chemistry for the analysis of saturated, unsaturated and polyunsaturated FAMEs, ranging in chain length from C4 to C24, with excellent peak shapes. Figure 2 shows the analysis of bacterial acid methyl esters (BAMEs) on the Equity-1. This C10-C20 mix contains hydroxyl-FAMEs and branched FAMEs in addition to saturated, unsaturated and polyunsaturated FAMEs. Again, excellent peak shape is observed for all analytes.
column: Equity-1, 15 m x 0.10 mm 1.0., 0.10 µm (28039-U)
oven: 100 °C, 50 °C/min. to 300 °C (1 min.)
inj.: 250 °C
det. FID, 300 °C
carrier gas: hydrogen, 50 cm/sec constant
injection: 0.2 µl, 200:1 split
liner: 4 mm I.D., split, cup design
sample: Supelco 37-Component FAME Mix (CRM47885), analytes at concentrations indicated in methylene chloride
- Butyric Acid Methyl Ester (C4:0) at 4 wt%
- Caproic Acid Methyl Ester (C6:0) at 4 wt%
- Caprylic Acid Methyl Ester (C8:0) at 4 wt%
- Capric Acid Methyl Ester (C10:0) at 4 wt %
- Undecanoic Acid Methyl Ester (C11:0) at 2 wt%
- Lauric Acid Methyl Ester (C12:0) at 4 wt %
- Tridecanoic Acid Methyl Ester (C13:0) at 2 wt%
- Myristic Acid Methyl Ester (C14:0) at 4 wt%
- Myristaleic Acid Methyl Ester (C14:1) at 2 wt%
- Pentadecanoic Acid Methyl Ester (C15:0) at 2 wt% .
- cis-10-Pentadecenoic Acid Methyl Ester (C15:1) at 2 wt %
- Palmitic Acid Methyl Ester (C16:0) at 6 wt%
- Palmitoleic Acid Methyl Ester (C16:1) at 2 wt%
- Heptadecanoic Acid Methyl Ester (C17:0) at 2 wt%
- cis-10-Heptadecenoic Acid Methyl Ester (C17:1) at 2 wt%
- Stearic Acid Methyl Ester (C18:0) at 4 wt%
- Oleic Acid Methyl Ester (C18:1n9c) at 4 wt%
- Elaidic Acid Methyl Ester (C18:1n9t) at 2 wt%
- Linoleic Acid Methyl Ester (C18:2n6c) at 2 wt%
- Linolelaidic Acid Methyl Ester (C18:2n6t) at 2 wt%
- g-Linolenic Acid Methyl Ester (C18:3n6) at 2 wt%
- a-Linolenic Acid Methyl Ester (C18:3n3) at 2 wt%
- Arachidic Acid Methyl Ester (C20:0) at 4 wt %
- cis-11-Eicosenoic Acid Methyl Ester (C20:1n9) at 2. wt %
- cis-11,14-Eicosadienoic Acid Methyl Ester (C20:2) at 2 wt%
- cis-8,11,14-Eicosatrienoic Acid Methyl Ester (C20:3n6) at 2 wt %
- cis-11,14,17-Eicosatrienoic Acid Methyl Ester (C20:3n3) at 2 wt %
- Arachidonic Acid Methyl Ester (C20:486) at 2 wt %
- cis-5,8,11,14,17-Eicosapentaenoic Acid Methyl Ester (C20:5n3) at 2 wt%
- Heneicosanoic Acid Methyl Ester (C21:0) at 2 wt%
- Behenic Acid Methyl Ester (C22:0) at 4 wt %
- Erucic Acid Methyl Ester (C22:1n9) at 2 wt%
- cis-13,16-Docosadienoic Acid Methyl Ester (C22:2) at 2 wt %
- cis-4,7,10,13,16,19-Docosahexaenoic Acid Methyl Ester (C22:6n3) at 2 wt %
- Tricosanoic Acid Methyl Ester (C23:0) at 2 wt%
- Lignoceric Acid Methyl Ester (C24:0) at 4 wt%
- Nervonic Acid Methyl Ester (C24:19) at 2 wt %
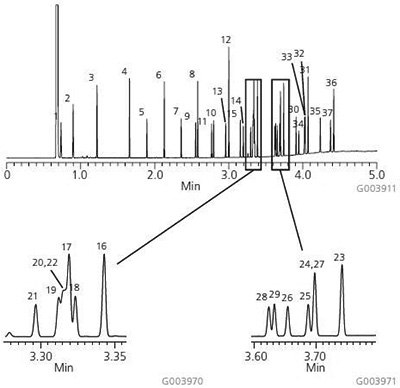
Figure 1. 37-Component FAME Mix on the Equity-1
column: Equity-1, 15 m x 0.10 mm I.D., 0.10 µm (28039-U)
oven: 175 °C, 30 °C/min. to 275 °C (1 min.)
inj.: 280 °C
det.: FID, 280 °C
carrier gas: hydrogen, 45 cm/sec constant
injection: 0.5 µL, 200:1 split
liner: 4 mm I.D., split, cup design
sample: Bacterial Acid Methyl Ester (BAME) Mix (47080-U), methyl ester derivatives of a naturally occurring mix of bacterial fatty acids, total concentration of 10 mg/mL in methyl caproate
- Methyl 2-hydroxydecanoate (2-OH-C10:0)
- Methyl undecanoate (C11:0)
- Methyl dodecanoate (C12:0)
- Methyl 2-hydroxydodecanoate (2-OH-C12:0)
- Methyl 3-hydroxydodecanoate (3-OH-C12:0)
- Methyl tridecanoate (C13:0)
- Methyl tetradecanoate (C14:0)
- Methyl 2-hydroxytetradecanoate (2-OH-C14:0)
- Methyl 3-hydroxytetradecanoate (3-OH-C14:0)
- Methyl pentadecanoate (C15:0)
- Methyl 13-methyltetradecanoate (i-C15:0)
- Methyl 12-methyltetradecanoate (a-C15:0)
- Methyl hexadecanoate (C16:0)
- Methyl 14-methylpentadecanoate (1-C16:0)
- Methyl 2-hydroxyhexadecanoate (2-OH-C16:0)
- Methyl cis-9-hexadecenoate (C16:19)
- Methyl heptadecanoate (C17:0)
- Methyl 15-methylhexadecanoate (i-C17:0)
- Methyl cis-9,10-methylenehexadecanoate (C17:0D)
- Methyl octadecanoate (C18:0)
- Methyl cis-9-octadecenoate (C18:19)
- Methyl trans-9-octadecenoate (C18:19) and
- Methyl cis-11-octadecenoate (C18:111)
- Methyl cis-9,12-octadecadienoate (C18:29,12)
- Methyl nonadecanoate (C19:0)
- Methyl cis-9,10-methyleneoctadecanoate (C19:0D)
- Methyl eicosanoate (C20:0)
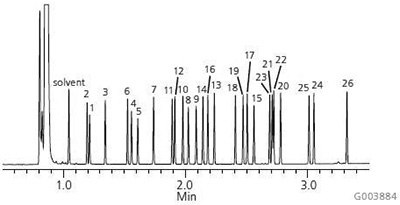
Figure 2.Bacterial Acid Methyl Esters (BAMEs) on the Equity-1
Note that both of the depicted chromatograms use Fast GC conditions: short column with narrow I.D., high carrier gas linear velocity, and rapid oven temperature programming. Columns with traditional dimensions (such as 30 m x 0.25 mm I.D., 0.25 μm) can also be used for these applications with equal success.
Chemical Standards
To assign identifi cation when performing the boiling point elution of fatty acid methyl esters for pattern recognition, standards of known reference must be used. To assist in confi rming identifi cation, Supelco offers several suitable standards. Selected standards can be found in the product listing at the end of this article. One standard is the Supelco 37-Component FAME Mix (CRM47885). This standard contains methyl esters of fatty acids ranging from C4 to C24, including key monounsaturated and polyunsaturated fatty acids, making this standard very useful to food analysts since it can be used to identify fatty acids in many different types of foods.
Highly Characterised Reference Oils are offered that can be used as controls or check samples, providing an excellent means of standardising applications and comparing results to others. AOCS Animal and Vegetable Reference Mixes are also available. Each quantitative mix is similar to the fatty acid distribution of certain oils, as specified in the AOCS reference mixes article on page 22, and conforms to the requirements of AOCS Method Ce 1-62.
Conclusion
Measuring and reporting of the fatty acid content of food is an important step that allows consumers the chance to establish a healthy dietary strategy. Equity-1 capillary GC columns and specially formulated chemical standards are quite suitable for the application shown in this article, GC analyses of FAMEs by boiling point elution.
This article, those in previous Reporter issues, and the sigma-aldrich.com/fame web page illustrate the breadth of specialised GC columns and the vast array of specially formulated chemical standards that are available for fatty acid and FAME analyses. With unsurpassed knowledge and product offerings, we are truly the total solution for obtaining superior products for the GC analyses of fatty acids from foods for nutritional needs.
References
To continue reading please sign in or create an account.
Don't Have An Account?