Tips & Tricks: Optimizing Immunoassay Protocols with Troubleshooting Advice
Looking for some tips and tricks on running immunoassays such as our MILLIPLEX® multiplex assays? Read on to get tips on reducing variability, correcting/preventing low bead counts, thorough plate washing, running antibody detection assays, immunoassay troubleshooting, and more.
Section Overview
- How to Run MILLIPLEX® Multiplex Immunoassays
- Tips for Reducing Variability
- How to Correct or Prevent Low Bead Counts
- Tips for Thorough Plate Washing
- Antibody Detection Assay Protocols
- Immunoassay Troubleshooting Guide
- Request Information
Note: Protocol procedures are optimized for best data results. Consequently, protocols can vary from kit to kit. Therefore, it is important to read the entire protocol before running an assay. The protocol information described below is for 96-well plate formats unless otherwise indicated.
How to Run MILLIPLEX® Multiplex Immunoassays
The key steps in the MILLIPLEX® assay protocol for the analysis of soluble biomarkers are:
- Collect serum, plasma, or other biofluid samples
- Prepare standards and samples and add them to plate
- Add conjugated beads and incubate at time and temperature specified in the protocol
- Wash beads
- Incubate with biotinylated-detection antibodies
- Add Streptavidin-PE (SAPE) and incubate
- Wash beads and resuspend in the appropriate buffer
- Acquire fluorescence on Luminex® instrument
Watch this quick video to see how to run MILLIPLEX® multiplex immunoassays.
Tips for Reducing Variability
- Ensure proper sample collection.
- To avoid low bead counts, thaw, vortex, and centrifuge all samples for 5-10 minutes at a minimum of 10,000 x g. Avoid or remove any debris, lipids, and cells that may be present.
- Centrifuge samples after thawing or if they appear turbid.
- This is especially recommended for plasma samples, cell/tissue lysates, or other sample types that are viscous or contain lipid/debris.
- For some sample types, the centrifugation may need to be repeated 2 or 3 times to completely clarify the supernatants.
- Ensure the proper mixing of samples and controls.
- Use appropriate pipetting techniques:
- Hold the pipette at the same angle each time.
- Use pipettes calibrated for values in the middle range (not extremes).
- Warm reagents to room temperature (20-25 °C) before mixing.
- For assays requiring overnight incubation in a cold room, warm reagents to room temperature on the second day as well.
- Cover the plate with a plate sealer before shaking.
- The plate shaker speed should be increased to agitate the plate at the highest speed that does not lead to splashing on the sealer.
- The day before running an assay, check the instrument.
- Is the instrument calibrated?
- Has it been maintained?
- Have fresh water prepared, calibrated and accurate pipettes, multichannel pipettes, and an orbital shaker or alternative.
- Confirm availability of a cold room or refrigerator with power access for the orbital shaker.
- When running samples in duplicate, a maximum of 38 samples can be run per 96-well kit (Figure 1). If using a MILLIPLEX® 384-well kit, a maximum of 182 samples may be run in duplicate.
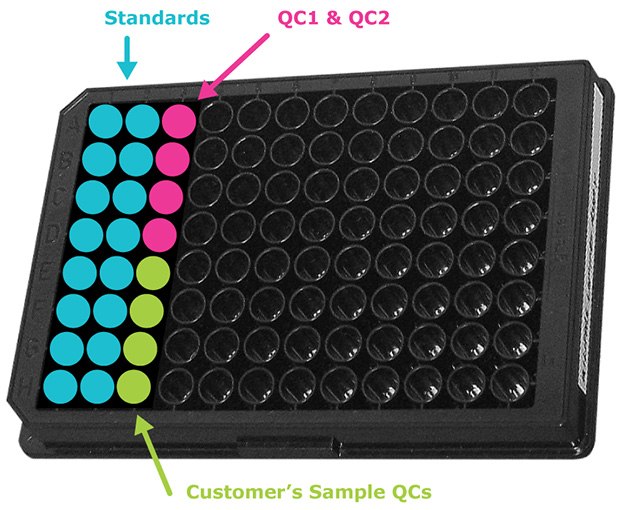
Figure 1.Sample 96-well plate map showing placement of standards, QCs, and samples. In this format, 38 samples can be analyzed in duplicate. 384-well plate set-up is similar, but with 182 samples in duplicate.
- To pre-wet the plate, use 200 µL Wash Buffer or assay buffer. Refer to the kit protocol for the appropriate buffer to use.
- If you accidentally use Wash Buffer instead of assay buffer for your assay, and if sample has not yet been loaded, remove Wash Buffer and replace it with assay buffer.
- If sample has been added to the plate with Wash Buffer, there is a potential for low recovery as it may not have the required protein concentration or protease inhibitors.
- Vortex all reagents well before adding them to the plate.
- When using frozen samples, it is recommended to thaw the samples completely, mix well by vortexing at a high setting, and centrifuge at a minimum of 10,000 x g prior to use to remove particulates.
- Be precise when adding samples, standards, and QCs to the plate.
- Pipette onto the sides of the wells.
- Be sure the appropriate amount of fluid is expelled from the pipette tips. Use fresh tips for each addition.
- For more precise fluid additions, use reverse pipetting techniques.
- For incubating assays overnight, a power supply must be available for the orbital shaker in a refrigerator or cold room.
- The plate shaker should be designed to hold a 96-well plate firmly, and it should reach at least 500 rpm. Do not use a gentle rocker or slow orbital mixer.
- If the plate shaker has been turned off during the night, shake again at room temperature for one hour before proceeding with the assay protocol.
- After overnight incubation of assays, remember to allow all reagents to warm to room temperature (20-25 °C) before use in the assay.
- Detection antibody cocktail and SAPE incubation times are critical. Do NOT exceed the dictated times as this may result in higher background signals. Also, do not under-incubate, as loss of signal dynamic range may occur.
How to Correct or Prevent Low Bead Counts
- Be sure to specify MagPlex® microspheres in the kit protocol for xPONENT® software or use the correct gate setting on Bio-Plex® software.
- Sample preparation: Thaw, vortex, and centrifuge samples at a minimum of 10,000 x g.
- Avoid or remove any debris, lipids, and cells layers that may be present.
- For samples known to be challenging (e.g., synovial fluid, saliva), you may increase wash steps after incubation with the beads.
- Resuspend beads in Wash Buffer instead of Sheath Fluid PLUS/Drive Fluid PLUS. However, the plate must be read within four hours.
- Add 1X Wash Buffer, which contains Tween® 20 reagent, to keep the beads from clumping or sticking.
- Store beads only in Sheath Fluid PLUS or Drive Fluid PLUS.
- During the wash steps while using a handheld magnet, decant the liquid, then gently blot the plate.
- When using a plate washer, check the settings to make sure the plate is soaking for 60 seconds, and the aspiration is not touching the bottom of the well.
- Warm the plate to room temperature after an overnight 4 °C capture antibody incubation step. Let the plate shake at room temperature for one hour.
- For MAGPIX® users, cleaning the instrument is critical.
- Special care should be taken to use the enhanced startup or washing procedures.
- There is an advanced cleaning method that includes sodium hydroxide (NaOH) and bleach.
- Washing between wells can also be selected during the plate reading.
- Cleaning the instrument regularly is important even if the instrument is not being used.
What If I Have Sticky Samples?
If your samples are particularly sticky, it can help to resuspend the beads in 1X Wash Buffer before reading the plate on the instrument. The detergents in this buffer can help with any aggregation that may occur. Note that the plate must be read within four hours.
What If the Protocol Does Not Include a Wash Step Between Detection and SAPE Incubations?
- If the detection antibody was aspirated off or poured off before adding SAPE to the well, it is possible to recover the assay using the following options:
- Add appropriate volume as indicated in the protocol of detection antibody and continue to follow the protocol.
- If additional detection antibody cocktail is not available, replace with assay buffer, add SAPE and continue to follow the protocol. Alternatively, you may add SAPE and continue to follow the protocol, keeping in mind that with both options the signal may be lower.
- Use the Sheath Fluid PLUS fluid, Drive Fluid PLUS (if using the MAGPIX® instrument), or if your samples are very “sticky,” use 1X Wash Buffer for the final resuspension before reading the plate.
- The plate should be read immediately (within 4 hours) after the assay is finished. If the plate cannot be read immediately, seal the plate, cover it with aluminum foil or an opaque lid, and store the plate at 2-8 °C for up to 72 hours on an orbital plate shaker, with samples brought up in Sheath Fluid PLUS/Drive Fluid PLUS. There may be a loss of sensitivity after 24 hours.
- Before reading the plate, agitate the plate on the plate shaker at room temperature for 10 minutes.
- Do not store processed samples in Wash Buffer.
- It is possible to run a portion of a plate initially, then reuse the plate with other samples later.
- Cover the wells that are not being used.
- Use precise volumes of reagents to ensure that enough remains to run the remaining wells at a later time.
- Store leftover reagents at appropriate conditions quickly after the first use (e.g., stock standard at -20 °C or lower).
- Remake standards for subsequent batches. Be sure to run a standard curve for each batch.
- When running subsequent batches, cover the previously used wells.
- The mix of beads may be used for one month if stored at 2-8 °C; stock standards should be stored at ≤ -20 °C for one month and -80 °C for more than one month. See specific kit protocol for directions on storage and use of leftover components.
- If using the same plate, keep the plate very clean. Alternatively, use a second plate for the remaining samples (for extra 96-well plates, use Product No. MAG-PLATE; for extra 384-well plates, use Product No. MAG384-PLATE).
Tips for Thorough Plate Washing
- Use the recommended orbital titer plate shaker (Corning® LSE™ Digital Microplate Shaker or equivalent)
- Very important: For incubating assays overnight, a power supply must be available for the orbital shaker in a refrigerator or cold room.
- The orbital titer plate shaker should be set at a speed to provide maximum orbital mixing without splashing of liquid outside the wells.
- For the recommended plate shaker, this is a setting of 5-7, which is approximately 500-800 rpm.
- However, orbital shakers vary. Your shaker can be calibrated by pre-wetting the plate with buffer and slowly increasing the speed until splashing occurs, then lowering the speed slightly. The shaker should be set at the highest speed allowable without splashing of the liquid.
- Use a Handheld Magnetic Separation Block (Figure 2)
- When ready to decant the liquid from the plate, the plate MUST be firmly attached to the magnet. To determine that the plate is attached firmly, listen for the click of the clasps.
- Grip the handheld separation block firmly.
- During the wash steps while using a hand magnet, decant the liquid, then gently blot the plate.
- When using a new magnet, check for space between the plate and magnet. Adjustments require a US Allen (hex) key to adjust the screws (not provided).
- Incomplete washing can adversely affect the assay outcome.
- All washing must be performed with the Wash Buffer provided.

Figure 2.Example of a handheld magnetic separator block for 96-well plates.
Antibody Detection Assay Protocols
- Antibody detection assays are qualitative (no standard included), with relative levels of detection presented as MFIs.
- Each kit includes four assay control beads, three with a different amount of immunoglobulin, providing varying levels of MFIs. One is a negative assay control bead that produces background-level MFIs.
- The assay control beads are not intended to be used for sample determination, only as assay controls for plate-to-plate consistency.
- It is recommended that experiment-specific positive and negative control samples are run to determine the appropriate assay cut-off MFI.
- Anti-human immunoglobulin conjugated to phycoerythrin is used in all antibody detection assays in place of detection antibody and SAPE steps.
- Each assay detects one immunoglobulin type: IgM, IgG, or IgA.
Typical Antibody Detection Protocol
- Prewet 96-well plate with 200 µL Wash Buffer and decant
- + 25 µL assay buffer
- + 25 µL sample (diluted serum or plasma); 25 µL assay buffer to background wells
- + 25 µL bead mixture
- Shake for 2 hours at room temperature or overnight at 4 °C. Refer to kit protocol for recommended incubation time.
- Wash beads x3 with Wash Buffer
- + 50 µL PE-Ig conjugate
- Shake 1.5 hours at room temperature
- Wash beads x3 with Wash Buffer
- + 150 µL Sheath Fluid PLUS/Drive Fluid PLUS and read on Luminex® instrumentation
Learn more about MILLIPLEX® Autoantibody Immunoassays in our “Multiplex Analysis of Autoantibodies” article.
Immunoassay Troubleshooting Guide
Table 1 is a troubleshooting guide for running MILLIPLEX® multiplex assays.
Request Information
Have more questions about MILLIPLEX® multiplex assays? Check out our FAQs or fill out the form by clicking on the button below.
For Research Use Only. Not For Use In Diagnostic Procedures.
To continue reading please sign in or create an account.
Don't Have An Account?