Multiplexed Evaluation of Soluble Immune-Checkpoint Molecules in Metastatic NSCLC Sera
Section Overview
- The Role of Immune Checkpoints in Cancer Immunotherapy
- PD-1/PD-L1 Pathway in NSCLC
- Methods
- Multiplexed Evaluation Results
- Conclusions
- Materials
Lung cancer remains the primary cause of cancer-related mortalities worldwide, a fact resulting from late diagnosis after widespread dissemination of the disease has occurred. PD-1/PD-L1 directed immune checkpoint inhibition therapy has changed the paradigm of cancer therapy and survivorship in this group with impressive, durable clinical benefits and low toxicity profiles. However, this specific approach is imperfect where only a subset of patients benefits from this therapeutic strategy. Multiple alternative immune checkpoint systems have been described as possible therapeutic options, though their potential impact in lung cancer remains undetermined.
In this study, a panel of 31 immune checkpoint molecules was profiled, consisting of soluble checkpoint molecules and immune regulators, using the MILLIPLEX® Human Immuno-Oncology Checkpoint Protein Panel 2 with pretreatment sera from 123 advanced-stage Non-Small Cell Lung Cancer patient samples receiving PD-1/PD-L1 targeted therapy.
Understanding the dynamics of these molecules and their association with clinical outcomes may help advance research in novel strategies for immune checkpoint inhibition therapy. Research evaluating soluble immune checkpoints in serum may help identify those likely to benefit from PD-1/PD-L1 immunotherapy and may reveal new immunotherapy targets for future investigation.
The Role of Immune Checkpoints in Cancer Immunotherapy
The immune system is an essential regulator of tumor biology with the potential of supporting or suppressing tumor growth and progression 1. Harnessing the immune system against cancer is the focus of several therapeutic approaches in cancer research. Immune checkpoint inhibitors (ICIs) are therapeutic monoclonal antibodies designed to disrupt inhibitory signals received by immune checkpoint regulatory molecules and show long-term survival benefits for some patients with metastatic melanoma, Non-Small Cell Lung Cancer (NSCLC), and other tumors 2-4. However, response to ICIs is not homogenous for all tumors, suggesting heterogeneity of immunoregulatory mechanisms in cancer 5. The coexistence of multiple checkpoints in the same tumor, for example, imposes a challenge for cancer immunotherapy and could be associated with resistance and treatment failure 6. Therefore, research to understand these resistance mechanisms may facilitate selecting patients who are most likely to benefit from current therapeutic strategies or indicate new therapeutic targets for future studies.
Immune checkpoint molecules are cell-surface proteins expressed on immune cells, mainly T cells, that regulate immune activation by various antigens, including tumor antigens. Immunotherapeutic agents harness the intrinsic immune response against tumor antigens by removing inhibitory mechanisms for T-cell activation upon interaction with antigen-presenting cells 7.
PD-1/PD-L1 Pathway in NSCLC
Programmed death receptor-1 (PD-1) and its ligand-1 (PD-L1) are the main targets for current immunotherapy agents approved for NSCLC, the most common subtype of lung cancer. However, the function and potential therapeutic value of numerous other immune checkpoints are not completely understood.
NSCLC is a leading cause of cancer mortality globally and is the second cancer that has benefitted from immune checkpoint therapy, especially in advanced stages 8, 9. Immunotherapeutic agents that target the PD-1/PD-L1 immune checkpoint pathway have been meteorically incorporated into the standard management of advanced-stage NSCLC after outstanding clinical benefits have been documented in a series of clinical trials. However, the overall response remains dismal for these patients and multiple resistance mechanisms have started to emerge, including the expression of alternate immune checkpoint molecules.
The research below evaluated circulating levels of 31 soluble immune checkpoints from patients receiving immunotherapy and correlated the levels to their clinical outcomes as a means to better appreciate the potential involvement of alternate ICI mechanisms in NSCLC.
Methods
Patient Sample Population
Serum was collected from 123 patients with advanced-stage NSCLC (IIIb-IV) that failed previous rounds of platinum doublet chemotherapy and before initiation of PD-1/PD-L1 directed immunotherapy (e.g., nivolumab, atezolizumab, pembrolizumab, or durvalumab). Clinical data were collected prospectively after written informed consent was obtained from each subject by the Rush University Cancer Center (RUCC) Biorepository. Clinical outcomes were assigned and calculated from the first immunotherapy administration date as follows:
- Progression-free survival (PFS) is the time elapsed from the treatment start date until the first tumor progression is encountered.
- Overall survival (OS) is the time elapsed between the treatment start date and death date or last date of follow-up with the patient known alive.
The Rush University Medical Center (RUMC) Institutional Review Board approved all protocols used in this study.
Serum Collection and Storage
Peripheral blood was collected in 10 mL red-top Vacutainers® immediately before initiation of PD-1/PD-L1 directed immunotherapy and processed using standard clinical laboratory methods. A portion of each serum sample used for the protein biomarker evaluations using the MILLIPLEX® immunoassays was supplemented with 25 µL/mL of Mammalian Protease Inhibitor Cocktail and 10 µL/mL of 0.5 M EDTA to minimize further proteolysis after processing. Aliquots were archived at -80 °C until testing, with no specimen tested subjected to greater than two freeze-thaw cycles.
Measurements of Serum Immuno-Oncology Biomarker Levels
Serum specimens were evaluated with 31 soluble checkpoint molecules and immune regulators using the MILLIPLEX® Human Immuno-Oncology Checkpoint Protein Panel 2. This panel is intended for research use only (RUO), and not for use in diagnostic procedures. This panel consists of the following targets:
|
|
All primary data points were collected on a Luminex® FLEXMAP 3D® system. Analyte concentrations were calculated from a 7-point curve using a five-parametric fit algorithm (xPONENT® v4.0.3 Luminex Corp., Austin, TX). All data met the minimum quality control thresholds defined in the kit protocol.
Biomarker Statistical Methods
Cutoff values in relation to survival parameters were determined using the “survminer” package in R program version 3.4. The association between the baseline biomarkers and the clinical outcomes was calculated using log-rank and Kaplan-Meier analysis using Graphpad v.8.3, and a p-value of 0.05 was used as a cutoff for statistical significance.
Multiplexed Evaluation Results
Analytical Performance
Analytes associated with immune regulation were quantified using the MILLIPLEX® Human Immuno-Oncology Checkpoint Protein Panel 2 in serum specimens representative of the pretreated advanced-stage NSCLC. The assays performed at a wide dynamic range with power of magnitude ranging from three to four. The precision was averaged at 94% with a high agreement between the samples and the standard curve. Table 1 shows the average coefficient of variation between the replicates, expressed as %CV (intra-assay precision).
Table 1. Average intra-assay precision values (expressed as %CV) between replicates. Coefficient of variance (CV) was calculated between the replicates of a standard curve, samples, and kit Quality Controls (QCs).
Performance with Patient Cohort: Progression-Free Survival
Based on the tested cohort, a significant association was observed between baseline soluble immune checkpoint molecules and the clinical outcomes. High baseline serum levels of CD40L, B7-H2/ICOSL, APRIL, B7-H5/VISTA, E-Cadherin, Galectin-1, Galectin-3, IDO1, Nectin-2, OX40/CD134, and Perforin were significantly associated with favorable progression-free survival time (p-values < 0.05). Kaplan-Meier curves of selected immune checkpoint molecules with cutoffs are depicted in Figure 1.
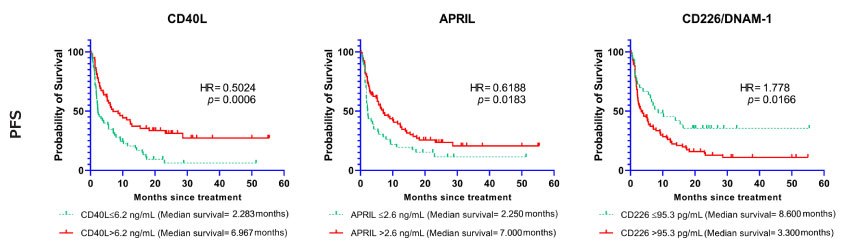
Figure 1.Soluble immune checkpoints (CD40L, APRIL, CD226/DNAM-1) associated with progression-free survival. Kaplan-Meier curves showing the association between selected biomarkers and progression-free survival (PFS). The curves represent the cases with baseline markers below or above the cutoff point.
Hazard ratios and confidence interval statistics (95% CI) are shown in detail in Table 2.
Table 2: Soluble immune checkpoints associated with progression-free survival. Association of analytical results with progression-free survival (PFS) with the optimized cutoff value indicated, number of cases below (N≤) and above (N>), median PFS below (low) and above (high) the cutoff, along with the log-rank p-value. Hazard ratios (HR) along with 95% confidence intervals (CI) are also provided.
Performance with Patient Cohort: Overall Survival
Many biomarkers were also associated with overall survival outcome. High baseline levels of CD40L, B7-H2/ICOSL, APRIL, B7-H5/VISTA, E-Cadherin, Galectin-1, Galectin-3, Granulysin, Nectin-2, OX40/ CD134, and Perforin were associated with longer overall survival (all p-values < 0.05). Lower baseline levels of B7-H3/CD276, 5'-NT/CD73, CD226/DNAM-1, FGL1/ Hepassocin, MICA, PVR/CD155, and Siglec-7 were associated with worse overall survival outcome (all p-values < 0.05). Hazard ratios and confidence interval statistics (95% CI) are shown in detail in Table 3.
Table 3: Soluble immune checkpoints associated with overall survival. Association of analytical results with overall survival (OS) with the optimized cutoff value indicated, number of cases below (N≤) and above (N>), median PFS below (low) and above (high) the cutoff, along with the log-rank p-value. Hazard ratios (HR) along with 95% confidence intervals (CI) are also provided.
Figure 2 shows Kaplan-Meier curves of overall survival in relation to baseline cutoff levels in selected immune checkpoint molecules.
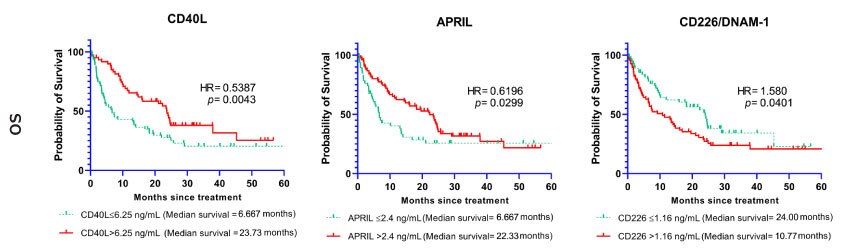
Figure 2. Soluble immune checkpoints (CD40L, APRIL, CD226/DNAM-1) associated with overall survival. Kaplan-Meier curves showing the association between selected biomarkers and overall survival (OS). The curves represent the cases with baseline markers below or above the cutoff point.
Conclusions
Soluble immune checkpoint molecules can be evaluated through multiplexing using the MILLIPLEX® Human Immuno-Oncology Checkpoint Protein Panel 2. Moreover, a significant association was shown between the circulating immune checkpoints molecules and the response pattern in NSCLC samples receiving anti- PD-1/PD-L1 therapy.
This research was conducted at Rush University Medical Center in Chicago, IL. Data was provided by Imad Tarhoni, M.D., Ph.D., Cristina L. Fhied, M.S., David Gerard, M.S., and Jeffrey A. Borgia, Ph.D. Departments of Cell & Molecular Medicine, Pathology, and the Rush Biomarker Development Core in 2020.
Vacutainers® is a trademark of Becton, Dickinson, and Company.
Materials
Related MILLIPLEX® Assays
For Research Use Only. Not For Use In Diagnostic Procedures.
References
To continue reading please sign in or create an account.
Don't Have An Account?