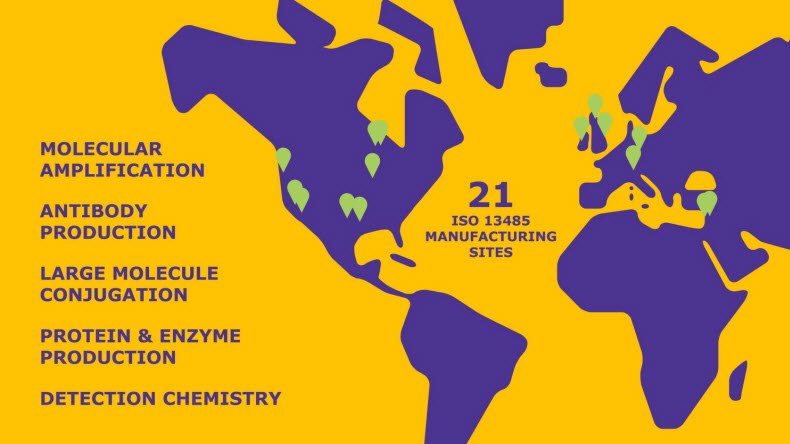
OEM & Contract Manufacturing Services for Life Sciences
Contract manufacturing for the diagnostics and life science industries provides a reliable, scalable solution for organizations that may not have the internal resources to manage production in-house. With robust expertise in raw material sourcing, quality systems, and regulatory compliance, our team delivers custom manufacturing services that ensure consistency, reliability, and customer satisfaction.
We partner with global leaders in diagnostics and life sciences, as well as emerging innovators bringing new technologies to market. Our end-to-end capabilities support the development and production needs of medical device manufacturers and diagnostic organizations worldwide. In addition to large-scale manufacturing, our OEM capabilities offer an extensive portfolio of custom diagnostic products, finished reagents, and critical raw materials designed for further manufacturing applications.
Through our OEM and contract manufacturing services, we provide flexible, scalable, and high-quality solutions that help life science companies accelerate development, meet regulatory requirements, and deliver transformative healthcare products to market.
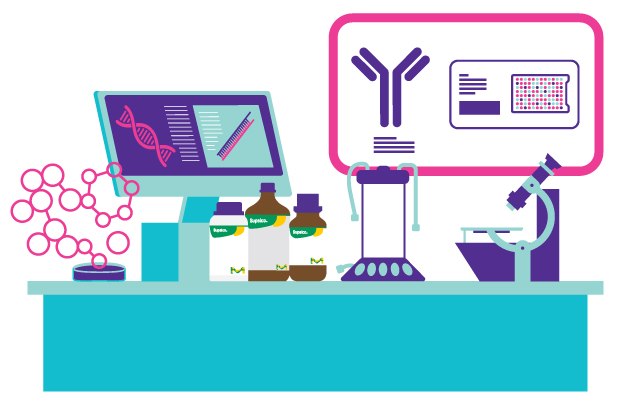
Image 1.Key technologies we offer for diagnostic assay OEM and Contract Manufacturing Services
Molecular Amplification and Antibody Production
In our OEM and Contract Manufacturing Services, we utilize advanced molecular amplification techniques to enhance the production of critical components. Our offerings include:
- GMP Oligos
- DNA/RNA enzymes
- Molecular-grade reagents
- Nucleic acid purification
- Kitting
Additionally, we specialize in monoclonal antibody production for various applications, including:
- Blood typing
- Infectious diseases
- Biomarkers
Conjugation and Detection Chemistry
Our expertise in conjugation is essential for integrating large molecules with beads and resins. In our OEM processes, we provide:
- Linkage chemistry
- Detection reagents
Our detection chemistry capabilities include:
- Chemiluminescence
- Colorimetric
- Fluorescence
Bio-organic Reagents and Protein/Enzyme Production
We offer a range of bio-organic reagents tailored for OEM applications, including:
- Detergents
- Resins
- Carbohydrates
- Buffers & solutions
Our protein and enzyme production capabilities encompass:
- Recombinant sources
- Plant and animal sourced
- Animal Source Free products
Related Resources
- Strategic Considerations When Outsourcing Production of In Vitro Diagnostics
The choice of whether to outsource production of an in vitro diagnostic (IVD) or invest and develop in-house capabilities is a foundational business decision. We highlight 10 important benefits of outsourcing and key considerations that should factor into the evaluation of an IVD manufacturing partner.
- Supply Chain Considerations When Developing or Manufacturing Your IVD Assay
Pandemic underscores importance of strong supply chain management for clinical diagnostic CMO partnerships.
- Diagnostic Assays Using Leading-Edge Technologies
Discuss challenges and strategies for IVD assay development, commercialization, and utilizing contract manufacturing partners for emerging technologies.
- Vetting a Contract Manufacturing Partner for Your Clinical Diagnostics Kit
Contract manufacturing aids IVD device commercialization, providing design, quality, and regulatory assistance for smoother processes.
- IVD Case Study: CRISPR-based Assay from Mammoth Biosciences
A case study describing how Mammoth Biosciences, a diagnostics company utilizing CRISPR gene editing collaborated with MilliporeSigma for manufacturing scale-up expertise and critical raw material supply chain security to ensure a successful launch and long-term success of their novel SARS CoV-2 test.
- Elypta's Contract Manufacturing Case Study
Elypta partners with us for standardized GAG quantification kits, meeting growing research assay demands along the regulatory path.
- Quality & Regulatory Management, ISO Certifications, and more...
Quality is embedded in everything we do, meaning we provide quality, compliance and business support in the most effective and efficient way for the entire portfolio of our life science business.
- Tech Article: Complete Solutions for PCR Assay Development
Fit-for-use products offer the quality, consistency & documentation necessary for every step of your IVD development and manufacturing process.
- Tech Article: Complete Solutions for IVD Chemiluminescent Immunoassay
Fit-for-use products offer the quality, consistency & documentation necessary for every step of your IVD development and manufacturing process.
Packaging Capabilities
Our extensive manufacturing capabilities in OEM and Contract Manufacturing ensure a secure supply chain and global footprint, providing reliability, consistency, and flexibility:
- Sterile filtration
- Hazardous materials
- Automated torque and weight checks
- Environmentally-controlled suites
- Molecular biology-grade environments
Powder Fill
We offer OEM powder fill solutions ranging from:
- 10 ug to 200 kg+
- Multiple sealing and capping options
- Pouching and tableting
Liquid Fill
Our automated filling capabilities support large batch volumes for IVD-grade liquid solutions, with fill volumes from:
- 10 µl to 20 L
- Large contract lyophilization capacity
- Environmentally-controlled filling
Formats
We provide a variety of packaging formats suitable for OEM products:
- Glass and plastic bottles
- Tubes and vials
- Boxes and Bags
- Cubitainers™
- Large Volume Totes
- Multiwell plates
- Custom labeling including Barcoding
Custom QC Testing
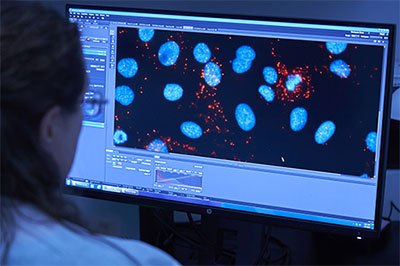
Image 2.QC testing analysis for an OEM or Contract Manufacturing Services for diagnostic assay
We offer key QC assay formats relevant to your OEM and Contract Manufacturing needs. Our analysis capabilities include:
- Molecular: qPCR, PCR, western blotting, Southern blotting, electrophoresis, nuclease contamination, bacterial growth, and cell component isolation kits (DNA, RNA, protein).
- Immunological: ELISA, western blotting, Ouchterlony double diffusion, cell staining, histology, serology.
- Functional: Plasmid purification, cloning, protein depletion, proximity ligation assays (Duolink®).
Additionally, we conduct specialized analyses, including:
- Endotoxins, hematology, enzymatic activity and contamination, cell culture, tablet testing (hardness, friability, and dissolution).
- Test method validation for niche QC needs and the ability to partner with external testing labs for esoteric tests.
- Functional testing of contract manufactured products on client platforms.
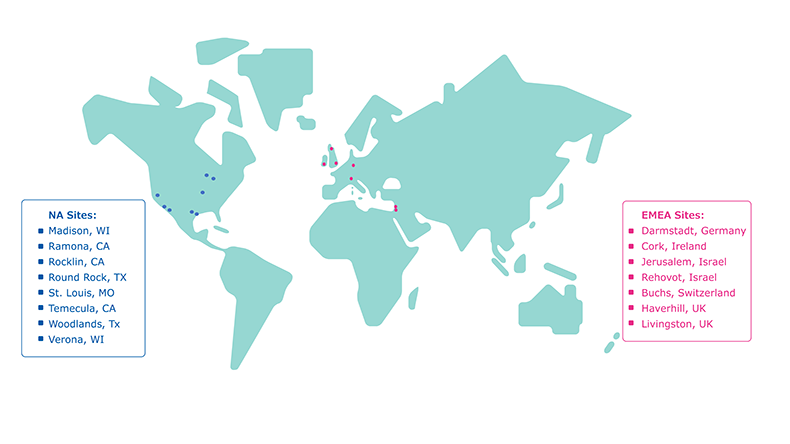
Image 3.NA and EMEA manufacturing sites for diagnostic assay components
Commitment to Quality
As an industry leader in OEM and Contract Manufacturing, our quality systems are designed to meet stringent regulatory requirements, including:
- ISO 9001:2015
- ISO 13485:2016
- 21 CFR 820
- 98/79 EC In vitro Diagnostics Directive
- ISO 14001:2015
Experienced Project Management to Secure Success
Our dedicated project managers are scientists who understand the technical, operational, and regulatory challenges of our client’s projects. For each client, we offer:
- Single Point of Contact for operations and project management
- Resources to help you navigate industry guidelines and regulations
- Support through your project life cycle
Steps for Success
Inquiry and Consultation
After receiving your inquiry, you will be contacted by one of our Business Development Specialists.
Acceptance
Project specifications are reviewed internally to determine feasibility and costing. Quote for project sent to you.
Creation of Project Team/Implementation
A cross-functional project team is assembled with expertise specific to project. Small-scale validation batched produced.
Full-Scale Implementation
We manufacture at scale to your specifications with your custom packaging/labeling for regional or global distribution.
To continue reading please sign in or create an account.
Don't Have An Account?