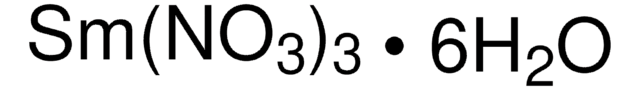GF55991076
Samarium
rod, 50mm, diameter 6.35mm, cast, 99%
Synonym(s):
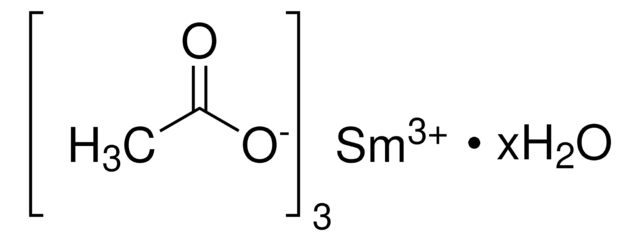
Samarium, SM007910
About This Item
Recommended Products
Assay
99%
form
rod
manufacturer/tradename
Goodfellow 559-910-76
resistivity
91.4 μΩ-cm, 0°C
L × diam.
50 mm × 6.35 mm
bp
1794 °C (lit.)
mp
1074 °C (lit.)
density
7.47 g/mL at 25 °C (lit.)
SMILES string
[Sm]
InChI
1S/Sm
InChI key
KZUNJOHGWZRPMI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Water-react 3
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Information
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service