Select a Size
About This Item
InChI key
YJVFFLUZDVXJQI-UHFFFAOYSA-L
InChI
1S/2C2H4O2.Pd/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
SMILES string
CC(O[Pd]OC(C)=O)=O
assay
≥99.9% trace metals basis
reaction suitability
core: palladium, reaction type: Buchwald-Hartwig Cross Coupling Reaction, reaction type: Cross Couplings, reaction type: Heck Reaction, reaction type: Hiyama Coupling, reaction type: Negishi Coupling, reaction type: Sonogashira Coupling, reaction type: Stille Coupling, reaction type: Suzuki-Miyaura Coupling, reagent type: catalyst
reaction type: C-H Activation
mp
216.3-223.7 °C (dec.)
Quality Level
Looking for similar products? Visit Product Comparison Guide
General description
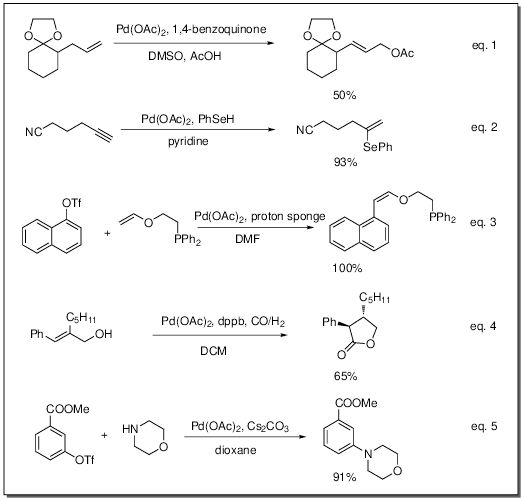
Application
- Formation of allylic acetates. (eq. 1)
- Hydroselenation of triple bonds. (eq. 2)
- Heck arylation of alkenes. (eq. 3)
- Cyclocarbonylation. (eq. 4)
- Buchwald-Hartwig amination reaction. (eq. 5)
For small scale and high throughput uses, product is also available as ChemBeads (924377)
signalword
Danger
hcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Sens. 1A
Storage Class
11 - Combustible Solids
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Which document(s) contains shelf-life or expiration date information for a given product?
If available for a given product, the recommended re-test date or the expiration date can be found on the Certificate of Analysis.
How do I get lot-specific information or a Certificate of Analysis?
The lot specific COA document can be found by entering the lot number above under the "Documents" section.
What reactions are Product 520764, Palladium (II) acetate, used in?
Palladium (II) acetate is used as a catalyst for a number of reactions, including: Suzuki coupling reactions, (See ChemFiles 2.1 and ChemFiles 4.2) vinylation, (i.e. the Heck Reaction), rearrangement of acyclic dienes (i.e. the Cope Reaction).Papers that cite the use of this reagent are listed below. 1. B.M. Trost Tet. 33, 2615, (1977)2. R.F. Heck Acc. Chem. Res. 12, 146, (1979)3. Tet. 62, 9002, (2006)4. Org. Lett. 8, 3311, (2006) abstract5. Organometallic News 2, 52, (2002)
What is Product 520764, Palladium (II) acetate, soluble in?
Palladium acetate is not water-soluble; it is organic soluble. Although Sigma-Aldrich does not test solubility of 520764 directly, it is expected to be soluble in most common organic solvents, like chloroform, methylene chloride and acetone, according to the manufacturer.
When using are Product 520764, Palladium (II) acetate, what can I use to remove traces of palladium that this catalyst may leave behind?
We would recommend using a metallic scavenger like the QuadraPureTM products. Product numbers 655422 and 657662 are designed to scavenge palladium ions and are effective in both acidic and basic conditions. The resins are capable of removing metallic contamination to very low levels and are ideal for pharmaceutical or fine chemical processing.
What is the difference between Product 520764, Palladium (II) acetate, and the palladium acetate in the form of a ChemDose® tablet?
The ChemDose® products offer a streamlined way to catalyze reactions. The catalyst is dispersed in an inert tablet matrix in specific millimolar and micromolar quantities. The tablets can decrease time spent on weighing/adding the catalyst to the reaction mixture. The tablets effectively give the same reaction yields with a controlled release of the catalyst. They are also extremely easy to remove after the reaction is complete. The inert tablet usually stays intact throughout the reaction. Product 684929 is the ChemDose® tablet that contains 2.0 μmol of palladium (II) acetate, while product#: 685593 is the 10μmol version. More information on these products can be found at the link below.
What atmosphere is Product 520764, Palladium (II) acetate, packaged under?
This material is packaged under a layer of nitrogen to reduce the chance of moisture contamination.
How do I find price and availability?
There are several ways to find pricing and availability for our products. Once you log onto our website, you will find the price and availability displayed on the product detail page. You can contact any of our Customer Sales and Service offices to receive a quote. USA customers: 1-800-325-3010 or view local office numbers.
What is the Department of Transportation shipping information for this product?
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
My question is not addressed here, how can I contact Technical Service for assistance?
Ask a Scientist here.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

