Select a Size
About This Item
Product Name
Tris(dibenzylideneacetone)dipalladium(0), 97%
SMILES string
[Pd].[Pd].O=C(\C=C\c1ccccc1)/C=C/c2ccccc2.O=C(\C=C\c3ccccc3)/C=C/c4ccccc4.O=C(\C=C\c5ccccc5)/C=C/c6ccccc6
InChI
1S/3C17H14O.2Pd/c3*18-17(13-11-15-7-3-1-4-8-15)14-12-16-9-5-2-6-10-16;;/h3*1-14H;;/b3*13-11+,14-12+;;
InChI key
CYPYTURSJDMMMP-WVCUSYJESA-N
assay
97%
form
powder
reaction suitability
core: palladium, reaction type: Buchwald-Hartwig Cross Coupling Reaction, reaction type: Cross Couplings, reaction type: Heck Reaction, reaction type: Hiyama Coupling, reaction type: Negishi Coupling, reaction type: Sonogashira Coupling, reaction type: Stille Coupling, reaction type: Suzuki-Miyaura Coupling, reagent type: catalyst
mp
152-155 °C (lit.)
Quality Level
General description
For small scale and high throughput uses, product is also available as ChemBeads (919772)
Application
- Application Guide for Palladium Catalyzed Cross-Coupling Reactions
- Synthesis of azepanes
- Synthesis of nanosized palladium phosphides upon interaction with white phosphorous
- Preparation of palladium triphenylphosphine carbonyl cluster complexes
- Precursor for synthesis of functionalized multiwalled carbon nanotube-palladium complexes used as catalysts for Heck coupling reactions
- Selective carbon-sulfur bond formation via addition of S-S and S-H bonds to alkynes
Reactant involved in:
- Catalyst for:
- Suzuki cross-coupling reactions
- PCN- and PCS-pincer palladium complex catalyzed tandem allylation
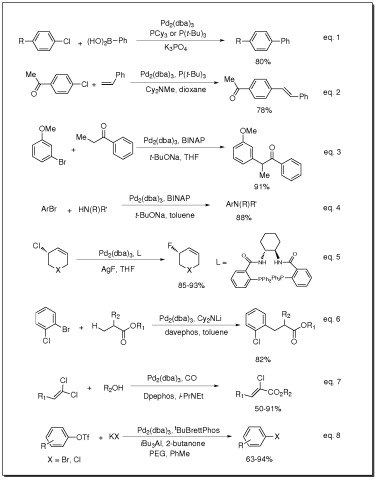
- Catalyst for Suzuki coupling of aryl chlorides (eq. 1)
- Catalyst for Heck coupling of aryl chlorides (eq. 2)
- Catalyst for arylation of ketones (eq. 3)
- Catalyst for Buchwald-Hartwig amination of aryl halides (eq. 4)
- Catalyst for fluorination of allylic chlorides (eq. 5)
- Catalyst for β-arylation of carboxylic esters (eq. 6)
- Catalyst for carbonylation of 1,1-dichloro-1-alkenes (eq. 7)
- Catalyst for conversion of aryl and vinyl triflates to aryl and vinyl halides (eq. 8)
- Pd source for enantioselective Tsuji Allylations
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 2 - Skin Sens. 1
Storage Class
11 - Combustible Solids
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
JosiPhos CyPF-tBu and palladium give catalyst for alkoxylation of activated heteroaryl halides with primary, secondary, and tertiary alcohols
Related Content
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
