125180
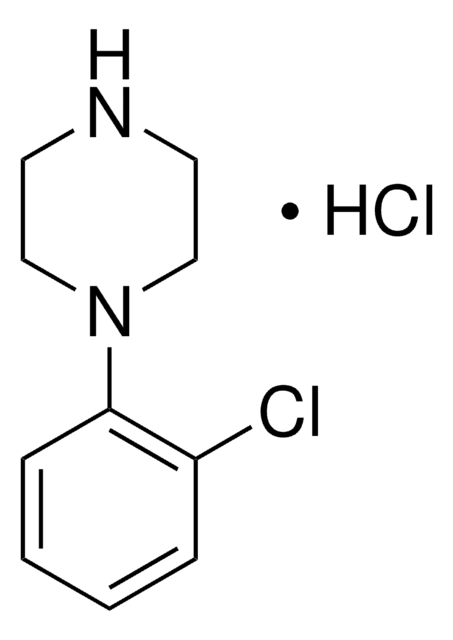
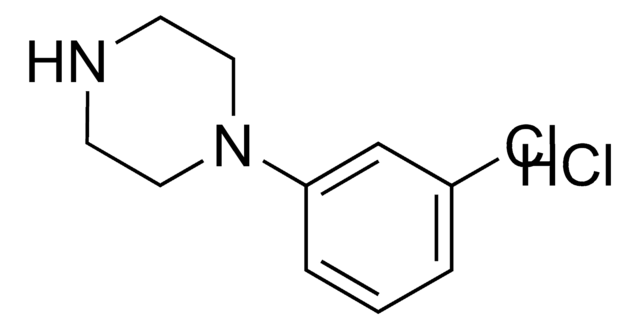
1-(3-Chlorophenyl)piperazine hydrochloride
99%
Synonym(s):
m-CPP
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H13ClN2 · xHCl
CAS Number:
Molecular Weight:
196.68 (free base basis)
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
210-214 °C (dec.) (lit.)
solubility
methanol: soluble
functional group
chloro
SMILES string
Cl.Clc1cccc(c1)N2CCNCC2
InChI
1S/C10H13ClN2.ClH/c11-9-2-1-3-10(8-9)13-6-4-12-5-7-13;/h1-3,8,12H,4-7H2;1H
InChI key
MHXPYWFZULXYHT-UHFFFAOYSA-N
Gene Information
rat ... Htr1a(24473)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1-(3-Chlorophenyl)piperazine hydrochloride is sold as Ecstasy mimic tablets and is the major metabolite of the antidepressant medications trazodone and nefazodone.
Application
1-(3-Chlorophenyl)piperazine hydrochloride (CPP) has been used in determination of designer piperazines in urine specimens using GC-MS and LC-ESI-MS. CPP has been used to investigate the in vitro efficacy of newly synthesized compounds such as fluoroquinolones (norfloxacine and lomefloxacine) against Philasterides dicentrarchi .
Biochem/physiol Actions
1-(3-Chlorophenyl)piperazine induces hypophagia in food-deprived and freely feeding rats. CPP has affinity for 11 neurotransmitter receptor binding sites in human brain membranes.
5-HT2c serotonin receptor agonist; metabolite of trazodone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Regulatory Information
新产品
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nan Zhang et al.
Journal of ethnopharmacology, 219, 23-30 (2018-03-17)
Cananga odorata essential oil, known as ylang-ylang essential oil (YYO), was commonly used in the aromatherapy for relaxation and mood adjusting use. In our previous study, YYO played anxiolytic effects on the mice in several behavioral tests that based on
Xiao Chen et al.
Behavioral and brain functions : BBF, 17(1), 4-4 (2021-05-20)
Obsessive-compulsive disorder (OCD) is a mental disease with heterogeneous behavioral phenotypes, including repetitive behaviors, anxiety, and impairments in cognitive functions. The brain regions related to the behavioral heterogeneity, however, are unknown. We systematically examined the behavioral phenotypes of three OCD
Horst Schicknick et al.
Cerebral cortex (New York, N.Y. : 1991), 18(11), 2646-2658 (2008-03-07)
Previous studies in the auditory cortex of Mongolian gerbils on discrimination learning of the direction of frequency-modulated tones (FMs) revealed that long-term memory formation involves activation of the dopaminergic system, activity of the protein kinase mammalian target of rapamycin (mTOR)
G A Kennett et al.
British journal of pharmacology, 103(4), 2016-2020 (1991-08-01)
1. 1-3(Chlorophenyl)piperazine (mCPP) (5 mg kg-1, i.p.) inhibited 2 h food intake in rats previously deprived of food for one day. Ten 5-hydroxytryptamine (5-HT) antagonists given s.c. opposed this hypophagic response. Calculated ID50 values correlated significantly with reported affinities (r
1-(m-chlorophenyl)piperazine (mCPP) interactions with neurotransmitter receptors in the human brain.
A Hamik et al.
Biological psychiatry, 25(5), 569-575 (1989-03-01)
The affinity of 1-(m-chlorophenyl)piperazine (mCPP) for 11 neurotransmitter receptor binding sites was determined in human brain membranes. mCPP is essentially equipotent at all 5-hydroxytryptamine (5-HT) receptor subtypes (IC50 values ranging from 360 to 1300 nM). The drug displays similar affinity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service