Detergents Use In Bioprocessing
Detergents, such as zwitterionic non-ionic surfactants, are used in biomanufacturing processes for a variety of applications including viral inactivation and cell lysis.
Looking For A Triton™ X-100 Alternative?
Triton™ X-100 (4-tert-octylphenol polyethoxylate), a non-ionic detergent, is commonly used for virus inactivation and cell lysis as it disrupts the lipid membrane of cells and enveloped viruses.
Triton™ X-100 is the main detergent templated in biomanufacturing processes, but as of January 2021, its unauthorized use has been prohibited in the European Union by the European Commission, due to its listing to REACH (Registration, Evaluation, Authorization and Restriction of Chemicals).
In response to the global implications of restrictions on Triton™ X-100 detergent, we have developed the Deviron® portfolio of sustainable detergents.
The Deviron® Detergent Portfolio as a Sustainable Triton™ X-100 Alternative
Our Deviron® portfolio of sustainable detergents should be considered as a primary alternative to Triton™ X-100 by all biologics manufacturers.
To address the need for alternatives to Triton™ X-100 that do not require a REACH authorization, we have launched the Deviron® C16 detergent and the Deviron® 13-S9 detergent.
KEY CONSIDERATIONS FOR DETERGENT SELECTION IN BIOPROCESSING
Several factors should be considered when evaluating detergents in biomanufacturing.
These include:
- Biodegradability (REACH* Compliance) and lack of harmful degradation products
- Viral inactivation (5 Log Reduction Value)
- Cell lysis efficiency
- Minimal impact on product functionality or quality
- Lack of process interference
- Effective removal by downstream operations
- Availability of sensitive detection method
- Manufacturability of high volumes as IPEC-PQG GMP quality material
- Impurity profile compatible with parenteral usage
*Registration, Evaluation, Authorisation and Restriction of Chemicals
Virus Inactivation for CHO Cell Harvest and Human Plasma Protein Purification
Viral safety is a major concern for biotherapeutic manufacturers.
- Cell-based processes may produce endogenous retroviral particles, and adventitious viruses can be introduced from contaminated source materials or during the manufacturing process.
- Human plasma-derived products are at risk of containing viruses, despite extensive screening of donation material.
Detergent-mediated viral inactivation is widely used in multiple biotherapeutic production processes as part of an overall virus safety strategy.
Our Deviron® portfolio shows efficient viral inactivation for both matrices with different model viruses and operating conditions.
Virus inactivation was assessed in mAb-containing CHO clarified harvest and human plasma matrices with XMuLV (Xenotropic Murine Leukemia Virus).
- The standard viral inactivation practice is described in the ASTM E3042-16.
- mAb processes >0.5%, no solvent and incubation time >60 min.
- Plasma processes typically utilize 1% detergent /0.3% solvent TnBP and longer incubation time (4-6 h).
Deviron® C16 and Deviron® 13-S9 demonstrate effective viral inactivation (LRV >5) in all conditions tested. Additional data with PRV and BVDV viruses are available.
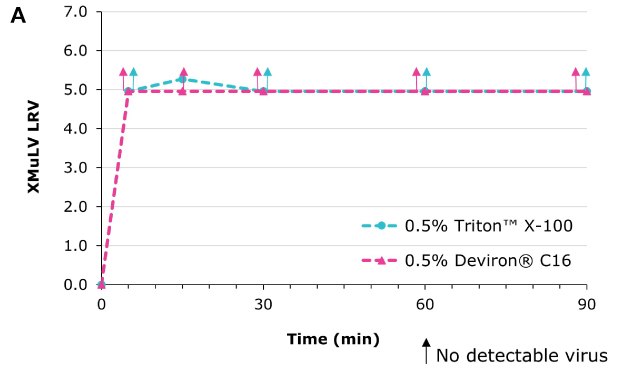
Deviron® C16 in CHO clarified harvest at 22°C
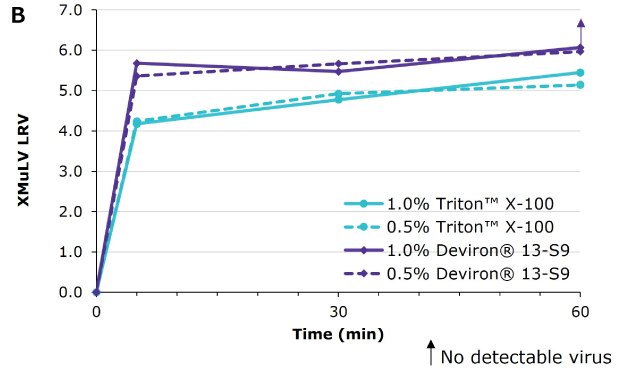
Deviron ® 13-S9 in CHO clarified harvest at 15°C
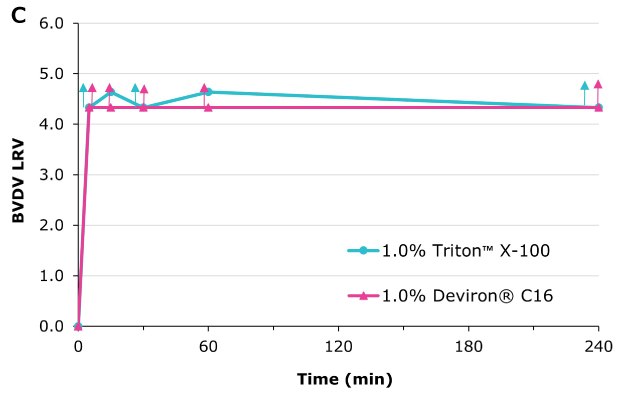
Deviron ® C16 in neat plasma with 0.3% TnBP at 22°C
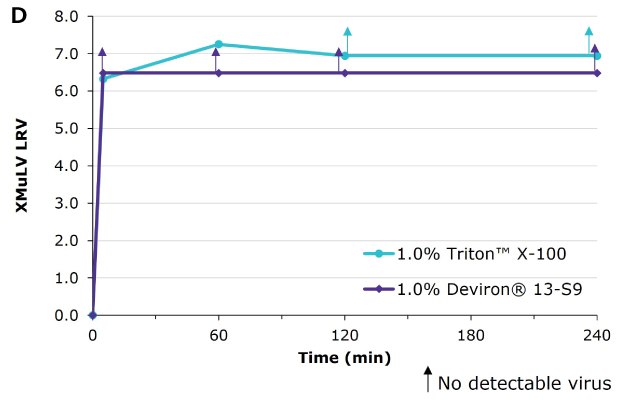
Deviron ® 13-S9 in cyro-poor plasma with 0.3% TnBP at 22°C compared to Triton™ X-100
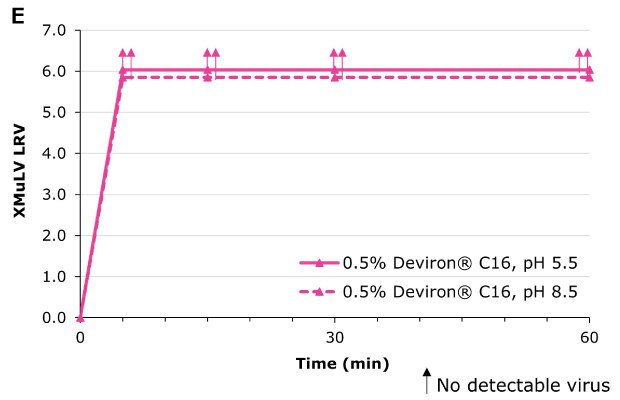
Deviron ® C16 in CHO clarified harvest at pH 5.5 and pH 8.5 at 22°C
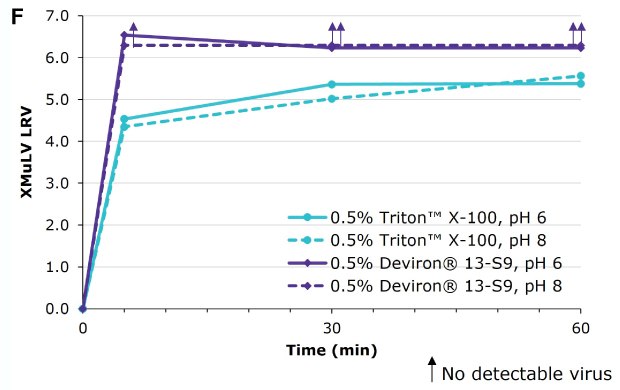
Deviron ® 13-S9 in IgG at pH 6 and pH 8 at 15°C
Cell Lysis for AAV Manufacturing
For biomanufacturing production of viral vectors used as therapeutics, a lysis step is needed to release the viral particles of interest. For Adeno Associated Virus (AAV) vectors used for gene therapies, host cells (HEK293 or Sf9 cells) used for production of viral vectors are often lysed using detergents like Triton™ X-100 or Polysorbates 20/80.
We introduce two new cell lysis options with our Deviron® detergent portfolio.
Cell lysis efficiency was assessed with HEK293 cells. After one day of cultivation, the cells were transfected with the selected plasmid polyethylenimine (PEl) complexation. After 72 hours of cultivation, the cells were harvested and lysate prepared according to the following procedure:
- Detergent concentration: 0.5% wt/vol
- Detergent: Tween™ 20 and Triton™ X-100 (benchmarks), Deviron® C16 and Deviron® 13-S9
- Nuclease: 25 U/mL Benzonase® with 2 mM MgCl2
- Lysis time: 2 hr
- Temperature: 37 °C
Following incubation, each sample was counted with ViCell and total cell count and viability determined.
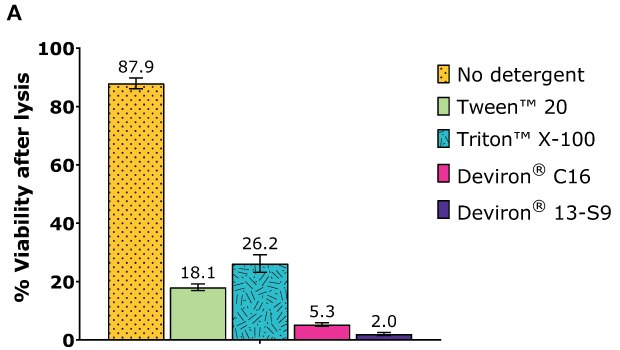
HEK293 Adherent cells – AAV2
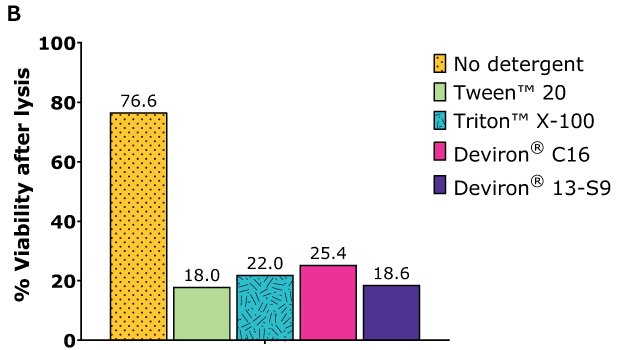
HEK293 suspension cells – AAV5
THE EMPROVE® PROGRAM FOR DETERGENTS
Roadmap for Choosing Fit-For-Purpose Raw Materials
As a supplier of thousands of raw materials for biologics manufacturing, we know the importance of quality and consistency. Our Emprove® program is designed to provide customers with the transparency and information needed to select fit-for-purpose raw materials and access to detailed documentation to meet regulatory requirements.
Provides detailed and transparent supply chain information and documentation to support risk assessments for critical raw materials used in manufacturing processes. Non-GMP but Manufactured utilizing GMP concepts and elements; ISO9001 or equivalent.
DEVIRON® 13-S9 Emprove® Expert
Addresses higher risk applications, where the lowest microbiological and endotoxin levels are of utmost importance. Released with IPEC-PQG GMP.
Related Resources
- Application Note: Deviron® Detergents for Viral Inactivation
Deviron® C16 and Deviron® 13-S9 Detergents for Solvent/Detergent Viral Inactivation of Plasma in Mobius® Single-Use Containers
- Brochure: The Deviron® Detergent Portfolio
Greener Alternatives to Triton™ X-100 for Biopharmaceutical Applications
- Poster: Sustainable Alternatives to Trition™ X-100 Detergent for Biomanufacturing
Deviron ® C16 and 13-S9 ® detergents are an effective sustainable alternative to Triton™ X-100 detergent.
- Hazard profile of Deviron ® 13-S9 detergent for GHS classification
The present summary collects the key toxicological and ecotoxicological data that were used for the GHS hazard classification of Deviron ® 13-S9 detergent.
- Flyer: Deviron® 13-S9 Detergent, EMPROVE® Expert
Aligning with two principles of Green Chemistry: Designing safer chemicals and Design for degradation.
To continue reading please sign in or create an account.
Don't Have An Account?