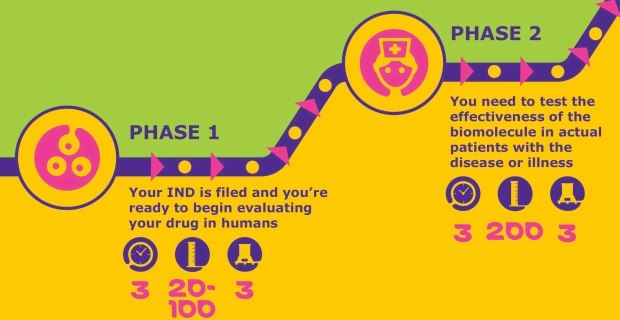
Phase I-II

Once the application for an investigational new drug (IND) is accepted, Phase I clinical trials can begin. During this development phase, the drug candidate will be tested on healthy individuals for safety.
Now is the time to develop, standardize, and scale drug manufacturing capabilities and ensure the proper analytics and quality control processes are in place capacity standardized
Once drug candidate safety is established, Phase II trials will evaluate efficacy. Success in this phase can lead to interest from partners and investors. In this stage, the focus is on scaling and further optimizing processes, and ensuring compliant endotoxin levels and sterility.
Featured Categories
Our ready-to-use, off-the-shelf cell line systems greatly reduce the time, cost, and risk of developing new cell lines for production. Whether you are producing antibodies, recombinant proteins, vaccines or viral vectors for gene therapies, our cell line platforms can accelerate your upstream progress.
Developing and implementing a clinical-scale process can be time-consuming and complex, requiring specification, sourcing, and integration of many components. With a clinical scale template, you can rapidly establish your own capabilities for production of pre-clinical, Phase I, and Phase II material.
Learn more about templated processes with these biopharm application guides for:
- Vaccines (including mRNA)
- Monoclonal antibodies (mAbs)
- Antibody drug conjugates (ADCs)
- Microbial
- Plasmid DNA (pDNA)
- Cell and Gene Therapy (CGT)
Access to the right resources helps you focus on discoveries and clinical candidates with the greatest potential to help patients in need. Select your stage of the development process to learn more or follow the product and service links for resources offering plug-and-play opportunities at the beginning of your startup creation.
Biotech Hub Resources Workflow

Discovery
Identifying the considerations, resources, and support you need to develop a new biologics candidate

Pre-clinical
Establishing safety and effectiveness for your Investigational New Drug (IND) application

Phase III and Manufacturing
Progressing from scale-up and tech transfer to quality production for trials and commercialization

Startup Programs
Connecting with resources and grant programs that can unlock your molecule’s potential

Regulatory
Navigating one of the world’s most regulated industries starts with a trusted guide
Visit our document search for data sheets, certificates and technical documentation.
Biotech Resources
- Biopharmaceutical Application Guide
Navigate the biopharmaceutical landscape with our application guide, providing resources and solutions for mAb, ADC, and mRNA processes.
- A Molecule’s Journey: Breaking Down Roadblocks to Clinical Success
A guidebook for biopharma executives navigating the complexities of clinical development. Learn how to successfully bring a molecule from the lab to the clinic.
- Quality and Regulatory Trends
Explore how we ensure high-quality products and services with a focus on compliance, risk management, and a strong code of conduct.
- Using a 3L Disposable Bioreactor to Increase Your Throughput Development Capacity
This webinar showcases the efficiency and economy of single-use bioreactors. Discover how using a 3L single-use bioreactor can enhance your throughput development capacity in therapeutic protein process development.
- Biosimilar Upstream Process Development – The Challenges and Promises
Explore the challenges and promises of biosimilar upstream process development. Learn how process changes impact product quality and achieve consistency in manufacturing.
- Mobius Single-Use Bioreactors Scalability: Bench to Clinical Scale
Discover how Mobius® Single-use Bioreactors enhance throughput development capacity, offering scalable solutions from bench to clinical scale.
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?

