Muscarinic Acetylcholine Receptors
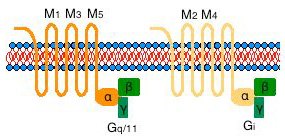
Muscarinic acetylcholine receptors are members of the superfamily of G protein-coupled receptors (GPCRs). They are relatively abundant and mediate many of the diverse actions of acetylcholine in the CNS, as well as throughout non-nervous tissues innervated by the parasympathetic nervous system. Five genes (m1-m5) encode muscarinic receptor proteins that contain the rhodopsin-like structural motif of seven transmembrane domains. They show strong sequence homology with each other and with related GPCRs within the transmembrane spanning domains, but each receptor also has unique amino acid sequences located at the amino end (extracellular), and in the third intracellular loop (I3). As with other biogenic amine receptors, much of the effector coupling specificity of these receptors resides in their intracellular domains, in particular the I3 loop.
The five muscarinic receptor subtypes are referred to as M1-M5. The odd-numbered receptors (M1, M3, M5) couple efficiently, through Gq/11, to activation of phospholipase C, which initiates the phosphatidylinositol turnover response. This leads to inositol trisphosphate-mediated release of calcium from the endoplasmic reticulum and to diacylglycerol-mediated activation of protein kinase C. As a consequence, depending on cell type, other cellular effectors may become activated subsequent to the stimulation of phosphatidylinositol turnover. In smooth muscle, muscarinic receptor (M3) activation of the phosphatidylinositol turnover response leads to elevation in cellular calcium and contraction. In glandular tissue, M3 receptor-mediated phosphatidylinositol turnover leads to hormone secretion. In brain, activation of post-synaptic M1 or M3 receptors often mediates “slow” neuronal excitability. One mechanism for this involves inhibition of calcium-regulated potassium channels, and this leads to an inhibition of the after-hyperpolarization phase of the neuronal action potential. Stimulation of post-synaptic muscarinic receptors themselves may not directly lead to action potentials, but commonly the activation of these muscarinic receptors enhances the neuron's response to excitatory input (this is sometimes called “neuromodulation”). Cortical and hippocampal muscarinic receptors transduce cholinergic input from the basal forebrain, in circuits believed to be important in the attentional aspects of cognition. In these brain areas, the predominant receptor subtypes are M1, M3 and M4. The striatum also expresses a mixture of muscarinic receptor subtypes, but the M4 subtype predominates. The M2 subtype is expressed at low levels in the telencephalon, most notably in the basal forebrain, and at relatively higher levels in the brainstem. The M5 subtype is expressed in brain at very low levels with a limited distribution.
The even-numbered muscarinic receptors (M2, M4) inhibit adenylyl cyclase activity via activation of the Gi class of G proteins. The M2 and M4 muscarinic receptors also activate G protein-coupled potassium channels, which leads to hyperpolarization of the plasma membrane of excitable cells. In nervous tissue, M2 and M4 receptors appear to frequently inhibit neuronal firing, and one or both of these subtypes are found on axon terminals where they inhibit neurotransmitter release (autoreceptors or heteroreceptors). The M2 muscarinic receptor inhibits adenylyl cyclase in smooth muscle and, as a consequence, opposes the effects of adrenergic innervation. In cardiac tissue, M2 muscarinic receptors activate G protein-coupled potassium channels to hyperpolarize the muscle, contributing to the slowing of the heart rate.
Besides their coupling to the well-established phosphatidylinositol and cyclic AMP effector systems, it is becoming increasingly clear that muscarinic receptors also couple to, or intersect with, signaling pathways that involve sequential activation of serine/threonine protein kinases, from which modulation of gene expression can result. For example, muscarinic receptors can activate certain MAP kinase pathways. Some components of the phosphokinase pathways that could conceivably be modulated by muscarinic receptors in vivo have the potential to enhance cell survival by upregulation of certain protection systems and/or blockade of apoptosis, or modulation of learning and memory.
Recent developments in muscarinic receptor biology include advances in the study of allosterism, including molecular work establishing where on the M1 receptor the novel agonist AC-42 binds. Other recent interesting discoveries include findings that muscarinic receptors regulate the proliferation of neural stem/progenitor cells and certain neurotransmitter uptake processes (e.g., GABA and norepinephrine transporters). Another recent study shows possible linkage between certain M2 receptor gene polymorphisms and alcohol dependence and depression. Thus, the therapeutic potential of muscarinic agonists seems to be expanding beyond the “replacement” for decreased acetylcholine in neurodegenerative disorders. Muscarinic receptor antagonists continue to be widely used in alleviating various symptoms of cholinergic hyperactivity.
Footnotes
a) Absolute selectivity for any muscarinic agent has so far not been achieved. The compounds listed are relatively, but not absolutely selective. Variations in their potencies/affinities may occur as a result of numerous factors, including tissue/species differences, variations in receptor densities and differences in the receptor/effector coupling efficiency. The reader is directed to the literature for detailed information concerning the pharmacological specificity of these compounds.
Abbreviations
AC-42: 4-n-butyl-1-[4-(2-Methylphenyl)-4-oxo-1-butyl]-piperidine hydrogen chloride
AF-DX 116: 11-([2-[(Diethylamino)methyl]-1-piperdinyl]acetyl)-5,11-dihydro-6-pyrido[2,3-b][1,4]benzodiazepin-6-one
AF-DX 384: 5,11-Dihydro-11-[2-[2-[(N,N-dipropylaminomethyl)piperidin-1-yl]ethylamino]-carbonyl]6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
CDD-0097: 5-Propargyloxycarbonyl-1,4,5,6-tetrahydropyrimidine
4-DAMP: 4-Diphenylacetoxy-N-methylpiperidine methiodide
L-689,660: 1-Azabicyclo[2,2,2]octane,3-(6-chloropyrazinyl)maleate
McN-A-343: 4-(N-[3-Chlorophenyl]carbamoyloxy)-2-butynyltrimethylammonium chloride
NMS: N-Methylscopolamine
QNB: Quinuclidinyl-a-hydroxydiphenylacetate; Quinuclidinylbenzylate
Similar Products
References
To continue reading please sign in or create an account.
Don't Have An Account?