HIC as a Capture Step
The objective of a capture step is to quickly bind the protein(s) of interest from the crude sample and isolate them from critical contaminants such as proteases and glycosidases. The target protein(s) are concentrated and transferred to an environment that will conserve potency/activity. Removal of other critical contaminants may also be achieved by careful optimization of binding conditions.
Focus is on capacity and speed in a capture step. It may be advisable to compromise on resolution in order to maximize the capacity and/or speed of the separation in this first step.
HIC media for capture steps should offer high speed and high capacity.
- Sepharose® Fast Flow (90 μm mean particle size) — good resolution at flows up to 300 cm/h.
- Use Sepharose® High Performance (34 μm particle size) HIC media for capture or scale-up when selectivity is satisfactory, high resolution is a priority and if lower flow rates (to compensate for a higher back pressure) are acceptable.
- Use SOURCE™ (15 μm mean particle size) if the required selectivity is not available in a medium of larger particle size.
Select start conditions that minimize binding of contaminants and so help to maximize the binding capacity for the target protein(s). This will facilitate a fast, simple step elution of the concentrated target protein(s).
Purification of recombinant human epidermal growth factor (h-EGF)
This purification represents a classical three-step purification process, using HIC-IEX-GF, for the purification of human epidermal growth factor (h-EGF) expressed as an extracellular product by Saccharomyces cerevisiae.

Fig 52 :Classical HIC-IEX-GF purification strategy.
The strategy was developed as follows: initial media screening experiments for the capture step were performed on four different HIC media (Figure 53). Phenyl Sepharose® 6 Fast Flow (high sub) was selected as giving the best selectivity for EGF, a high binding capacity with low back pressure.
Media characteristics for EGF purification
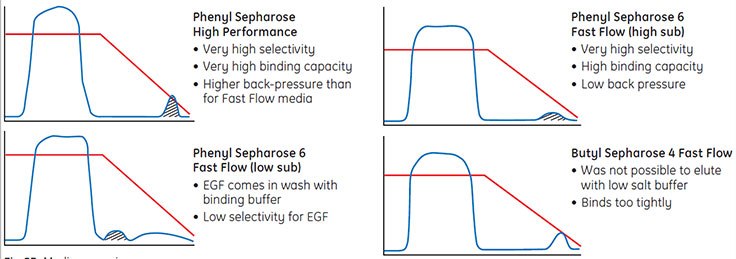
Fig 53 :Media screening.

Fig 54 :Development and scale-up of the capture step.
As Figure 54 shows, development work was performed at a small scale using a step elution on XK columns packed with Phenyl Sepharose® 6 Fast Flow (high sub) and then scaled up to production scale using a BPG 300/500 column.
A high-resolution, anion exchanger, Q Sepharose® High Performance, was chosen for intermediate purification in order to reach a high degree of purity in the second step (> 96%).
Gel filtration on Superdex™ 75 prep grade was selected for final polishing in order to achieve a high final purity by separating polymers and unwanted buffer salts from the EGF product.
The start material was clarified cell culture supernatant supplied by Chiron-Cetus Corp., Emeryville, USA. Concentration of EGF in the start material was 0.018 mg/ml and the overall protein content was 63 mg/ml. This three-step procedure gave a product purity of 99% as determined by RPC, and an overall yield of 73%.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?