Cleavage Enzymes for Fusion Protein Purification
After purifying epitope-tagged fusion proteins using agarose or magnetic bead resins, cleavage enzymes are used to isolate untagged, intact proteins of interest.
Cleavage Enzymes
Cleavage enzymes degrade proteins through cleaving peptide bonds. Use the table below as a guide to choose the cleavage enzyme that matches your affinity purification scheme based on the sequence they recognize.
Click on the cleavage enzymes below to get more information.
Factor Xa
Factor Xa is an endopeptidase that cleaves proteins at the sequence Ile-(Glu or Asp)-Gly-Arg-X, where X is any amino acid other than proline or arginine. Factor Xa cleaves at the C-terminal end of the Arg residue and is frequently used to remove polyhistidine tags during protein affinity purification on nickel columns.
The Factor Xa Cleavage Capture Kit is designed for highly-specific cleavage of fusion proteins followed by convenient affinity-based capture and removal of Factor Xa. After cleavage of the target protein, Factor Xa is removed with greater than 99% efficiency from the reaction by affinity capture on Xarrest™ Agarose.
Also, Restriction Grade Factor Xa is a highly purified preparation isolated from bovine plasma and activated with Russell's viper venom. This preparation is purified to near homogeneity and shows no secondary cleavage from contaminating proteases. Figure 1 shows an example of Factor Xa cleavage.

Figure 1.10 µg of Factor Xa cleavage control protein (CCP, lane 1) was digested with increasing amounts (0.03 µg to 0.5 µg per reaction) of Factor Xa (lanes 2-5). SDS-PAGE (4-20% gradient gel) followed by staining with Coomassie® Blue showed the two expected proteolysis products (~35 kDa and ~17 kDa).
Enterokinase
Enterokinase specifically cleaves proteins at the specific sequence, Asp-Asp-Asp-Asp-Lys-X, with X being any amino acid other than proline, at the C-terminal end of the lysine. Since this sequence is included in the widely used FLAG® affinity tag, enterokinase is a commonly used enzyme for protein affinity purification.
The Enterokinase Cleavage Capture Kit is designed for highly-specific cleavage of fusion proteins followed by rapid, affinity-based capture and removal of enterokinase.
Also, Recombinant Enterokinase (rEK) is a highly purified preparation of the catalytic subunit of bovine enterokinase, a serine protease which recognizes the identical cleavage site as the native enzyme and has similar enzymatic activity.
Thrombin
Restriction-grade and biotinylated thrombin enzymes are ideal for highly efficient cleavage of fusion proteins.
Restriction Grade Thrombin is qualified to specifically cleave target proteins containing the recognition sequence LeuValProArg↓GlySer. The preparation is functionally tested for activity with fusion proteins and is free of detectable contaminating proteases. Thrombin is supplied with 10X Thrombin Cleavage Buffer and a Cleavage Control Protein.
Biotinylated Thrombin is identical in activity to Restriction Grade Thrombin but has covalently attached biotin for easy removal of the enzyme from cleavage reactions using immobilized streptavidin. Our Thrombin Cleavage Capture Kit not only includes biotinylated thrombin and immobilized streptavidin, but also all the required buffers and filters for complete, convenient recovery of cleaved protein. Figure 2 shows an example of biotinylated thrombin cleavage.
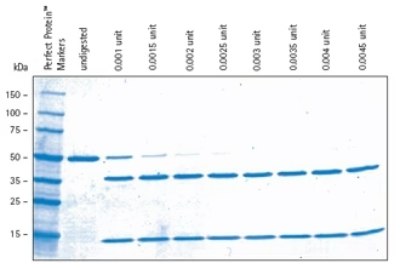
Figure 2.Biotinylated thrombin cleavage with respect to amount of thrombin added. The indicated amounts of biotinylated thrombin were used to cleave 2 μg of Cleavage Control Protein in an overnight digestion. Samples were analyzed by SDS-PAGE (4-20% gradient gel) followed by staining with Coomassie® Blue. The 0.0045-unit lane represents a 2.25-fold overdigestion.
HRV 3C Protease
Recombinant type 14 3C protease from human rhinovirus (HRV 3C) is a highly-purified, recombinant 6XHis-tagged enzyme, which recognizes the cleavage site LeuGluValLeuPheGln↓GlyPro. The small, 22-kDa size of the protease, with optimal activity at 4 ºC, high specificity, and His•Tag® fusion make HRV 3C protease an ideal choice for rapid removal of fusion tags, even from labile target proteins. Figure 3 shows an example of how this protease cleaves fusion proteins.
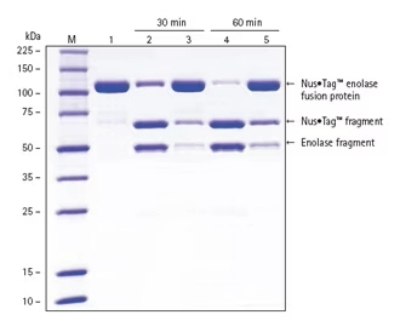
Figure 3.HRV 3C Protease cleaves fusion proteins more efficiently (lanes 2 and 4) compared to cleavage with a competitor’s protease (lanes 3 and 5). Experimental Methods: Using a 1:100 (w/w) ratio of protease:target protein, 500 μg of purified Nus•Tag™ enolase fusion protein was incubated in parallel 500 μL reactions at 4 °C. The reactions were quenched by adding equal volume 4X SDS Sample Buffer and then immediately placing the samples into a water bath at 75 °C for 5 min. Lane M – Perfect Protein™ Markers, 10-225 kDa, lane 1 - 3 μg purified Nus•Tag™ enolase fusion protein, lane 2 - 3 μg Nus•Tag™ enolase fusion protein with 30-min HRV 3C protease reaction, lane 3 - 3 μg Nus•Tag™ enolase fusion protein with 30-min competitor protease reaction, lane 4 - 3 μg Nus•Tag™ enolase fusion protein with 60-min HRV 3C protease reaction, lane 5 - 3 μg Nus•Tag™ enolase fusion protein with 60-min competitor protease reaction.
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?