Horseradish Peroxidase (HRP) Enzymes
Our horseradish peroxidase is specially selected and cultivated from horseradish roots, and our growers have continuously implemented a long-standing history of process improvements that ensure quality, consistency and optimized yield. For decades, we have manufactured peroxidase at our St. Louis ISO 9001:2000 production facility in multi-kilo batch sizes. While the supply of horseradish in other parts of the world has been an issue, the North American horseradish supply has grown stronger. Our St. Louis production facility overlooks an area of the Mississippi Valley known as the American Bottoms, which produces 60% of the world's horseradish.
The production facility has an experienced well-trained workforce that follows rigorous operating procedures for processing, equipment, cleaning, testing and packaging operations within robust quality and quality assurance systems.
Our peroxidase is recognized around the world as the industry standard for diagnostic manufacturing and laboratory scale research applications. We offer a variety of products based on purity, isoform content, and covalent modifications, to accommodate established applications as well as basic research needs.
Read more about
Related Products
Horseradish Peroxidase Function and Activity
Specific activity is expressed in terms of pyrogallol units. One pyrogallol unit will form 1.0 mg purpurogallin from pyrogallol in 20 sec at pH 6.0 at 20° C, unless otherwise indicated in the listing. This purpurogallin (20 sec) unit is equivalent to approx. 18 µM units per min at 25° C.
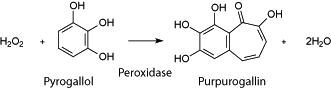
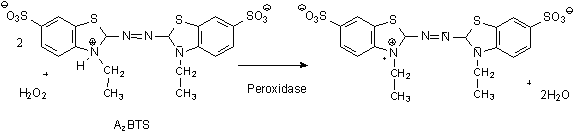
ABTS units are another commonly used unit definition. One ABTS unit will oxidize 1 µmole of ABTS per minute at 25 °C at pH 5.0. Using ABTS as the substrate, approx. four times the activity is observed over the pyrogallol system.
The RZ (Reinheitszahz) is the absorbance ratio A403/A275 determined at 0.5–1.0 mg/mL in deionized water. It is a measure of hemin content and is not necessarily indicative of enzymatic activity.
Product Characteristics for Native Horseradish Peroxidase
Extinction Coefficient: EmM = 100 when measured at 403 nm.1
Molecular Weight: (approx. 44 kDa)
Includes the polypeptide chain (33,890 Daltons), hemin plus Ca2+ (approx. 700 Daltons), and carbohydrate (9400 Daltons).3
Isoelectric Point: Isozymes range from 3.0 – 9.0
At least seven isozymes of HRP exist.2
Horseradish peroxidase (HRP) is isolated from horseradish roots (Amoracia rusticana) and belongs to the ferroprotoporphyrin group of peroxidases. HRP is a single chain polypeptide containing four disulfide bridges. It is a glycoprotein containing 18% carbohydrate. The carbohydrate composition consists of galactose, arabinose, xylose, fucose, mannose, mannosamine, and galactosamine, depending upon the specific isozyme.2
Substrate Specificity
HRP readily combines with hydrogen peroxide (H2O2), and the resultant [HRP–H2O2] complex can oxidize a wide variety of chromogenic hydrogen donors. It can also utilize chemiluminescent substrates such as luminol and isoluminol, and fluorogenic substrates such as tyramine, homovanillic acid, and 4–hydroxyphenyl acetic acid.
The Enzyme Explorer's Substrate Index provides links to several chromogenic and chemiluminescent hydrogen donors used to assay peroxidase activity.
Inhibitors
The following compounds are inhibitors of horseradish peroxidase: sodium azide, cyanide, L–cystine, dichromate, ethylenethiourea, hydroxylamine, sulfide, vanadate, p–aminobenzoic acid, Cd+2, Co+2, Cu+2, Fe+3, Mn+2, Ni+2, Pb+2.4
pH Dependence
The pH optimum of HRP is in the range of 6.0 to 6.5. Activity at pH 7.5 is 84% of the maximum. The enzyme is most stable in the pH range of 5.0 to 9.0.5
Applications
Horseradish peroxidase is widely used as a label for immunoglobulins in many different immunochemistry applications, including ELISA, immunoblotting and immunohistochemistry. HRP can be conjugated to antibodies by several different methods, including glutaraldehyde, periodate oxidation, through disulfide bonds, and also via amino and thiol directed cross-inkers. HRP is the most desired label for antibodies since it is the smallest and most stable of the three most popular enzyme labels (HRP, alkaline phosphatase, and B-galactosidase), and its glycosylation leads to lower non-specific binding.6 Protocols describing the glutaraldehyde and periodate conjugation methodologies can be reviewed in Harlow, E. et al.7
We offer several chromogenic and chemiluminescent detection reagents for blotting, histology and ELISA applications.
We also offer a complete line of HRP conjugated antibodies.
Preparation Instructions
The choice of solvent will depend on the intended application. The powdered enzymes are soluble water or 0.1 M phosphate buffer, pH 6 (10 mg/mL). The suspension (P6140) may be diluted in water.
Storage/Stability
The powdered peroxidases should be refrigerated at 2-8 °C. Freezer storage is also acceptable but not required. The suspension (P6140) should be stored at 2-8 °C. If properly stored, these products have a shelf life of at least two years.
RZ Profile
RZ (Reinheitszahl): the absorbance ratio A403/A275. It is a measure of hemin content of the peroxidase, not enzyme activity. Even preparations with a high RZ value may have low enzymatic activity. For conjugating proteins such as antibodies to peroxidase, choose a peroxidase with an RZ value of at least 3.0.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?