Water for Nuclease-sensitive Molecular Biology Studies
Nucleases play a critical role in many biological processes and can be found in many cell types. These enzymes can cleave the phosphodiester bonds between nucleotides of nucleic acids. They include ribonucleases (RNases), specific for RNA degradation, and deoxyribonucleases (DNases), specific for DNA degradation. Nucleases can degrade nucleic acids from an extremity of the strand (exonucleases) or from the middle of the strand (endonucleases). In situ, RNases degrade foreign nucleic acids, regulate gene expression by degrading mRNA, generate mature RNA and can even have antitumor activity.
In the laboratory, nucleases can be problematic as they may degrade samples, extracts or PCR products by cleaving DNA and RNA strands into smaller fragments. Unfortunately, they are ubiquitous in the lab. For example, several classes of these enzymes exist in bacteria, with various selectivity and activity. They are extremely active, and only a small amount is sufficient to degrade nucleic acids present in samples.
WHY IS NUCLEASE-FREE WATER NEEDED IN MOLECULAR BIOLOGY RESEARCH?
To ensure against sample loss, lack of reproducibility or experimental failure, it is important to keep samples, buffers, plasticware and glassware, benches, and all containers free of nucleases at all times. Nuclease-free water (also known as ‘PCR water’ or ‘molecular biology grade water’) is therefore recommended to prepare samples, reagents and buffers for molecular biology assays and experiments involving nucleic acids.
How to prepare nuclease-free, molecular biology grade water
Nuclease-free ultrapure water is a major component in buffers and reagents used in molecular biology experiments involving DNA or RNA, such as nucleic acid extraction, PCR, cloning, gene editing, and sequencing. It can be prepared in several ways.
Chemical treatment with DEPC
Nuclease-free water has long been prepared via DEPC (diethylpyrocarbonate) treatment.1 DEPC reacts with the nitrogen atoms of the histidine residues of the RNase active site, thereby inactivating it. Following the inactivation step, the solution is autoclaved to eliminate any excess DEPC by hydrolysis.
This method, while efficient and time-tested, has several drawbacks:
Both chemical inactivation and autoclaving steps generate by-products such as ethanol and CO2, which impair water quality. Ethanol causes organic contamination (TOC) and may generate unwanted by-products in later experimental steps. CO2 reacts with water to generate carbonic acid and bicarbonate (Figure 1).
DEPC has a strong affinity for adenosine, and residual DEPC traces remaining in solution may compromise experimental results (blots, PCR reactions, etc.) by modifying adenosine nucleotides. It may also affect some downstream enzymatic reactions since it can react with -NH, -SH, or -OH groups (e.g., Cys, His, Lys, Ser, Thr, and Tyr residues in active sites).2
Since DEPC reacts with nitrogen, buffers based on amine containing compounds, such as HEPES and Tris, cannot undergo DEPC treatment.
DEPC treatment raises some safety concerns since it can easily hydrolyze and is toxic. In addition, the reaction and autoclaving steps are time consuming and require energy and water.
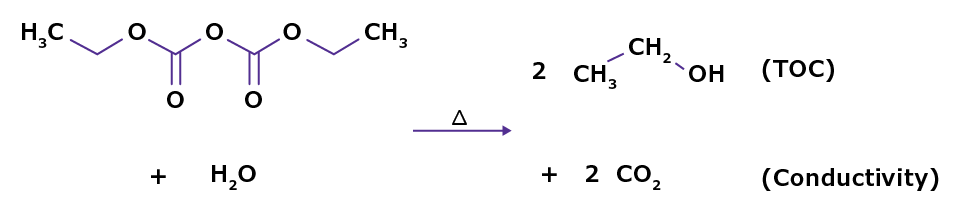
Figure 1. Degradation of DEPC leads to water contamination.
Ultrafiltration efficiently delivers nuclease-free water
In water, nucleases mainly originate from bacteria. The molecular weight of bacterial RNases may vary significantly. For instance, RNase I has a molecular weight of 27 000 Da, while RNase III has two 26 500 Da subunits.
Ultrafiltration is a membrane-based technology that removes nucleases by a filtration process. Ultrafilters are depth filters with nominal weight cut-offs ranging from 5 000 Da to 100 000 Da. By placing a carefully selected ultrafilter at the point-of-use outlet of the water purification process, it is possible to obtain nuclease-free water on demand (Figure 2). The water delivered is RNase-free and does not require DEPC treatment or autoclaving.
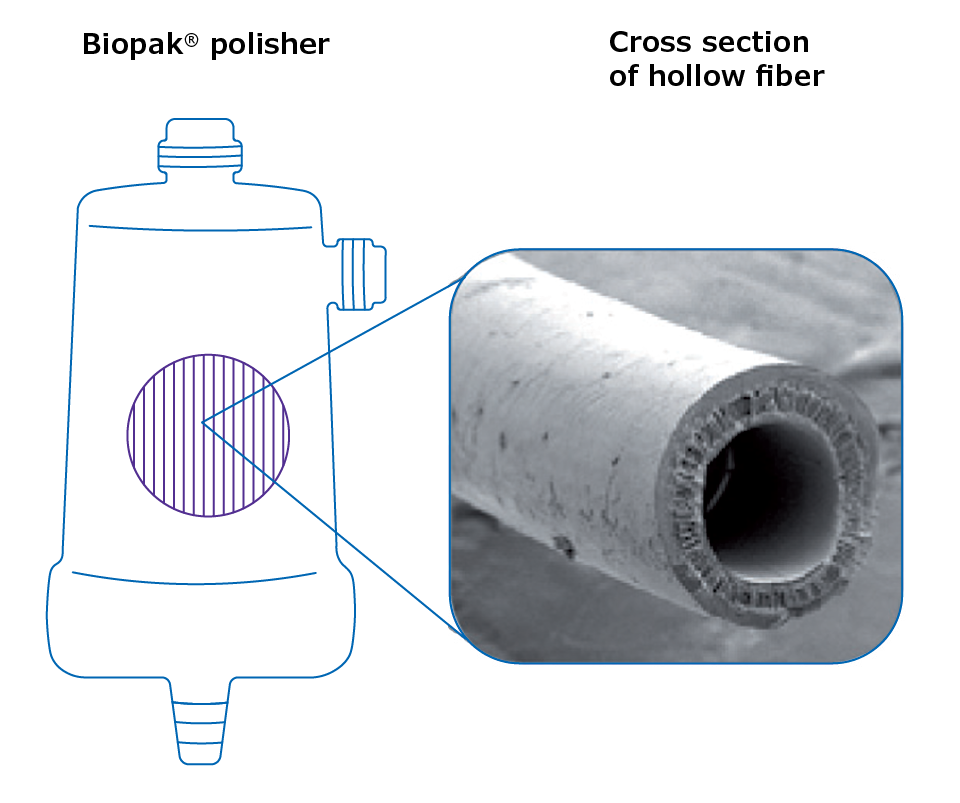
Figure 2. Scanning electron microscopy (SEM) photo showing a cross-section of one of the ultrafiltration polysulfone hollow fibers contained in a Biopak® polisher.
To demonstrate the efficiency of ultrafiltration, RNase was added to ultrapure water, and the solution was filtered through a Biopak® ultrafilter. Figure 3 demonstrates the efficiency of RNase removal by the ultrafiltration cartridge. Ultrafiltration was fully efficient over a three-month period and large quantities of nucleases.
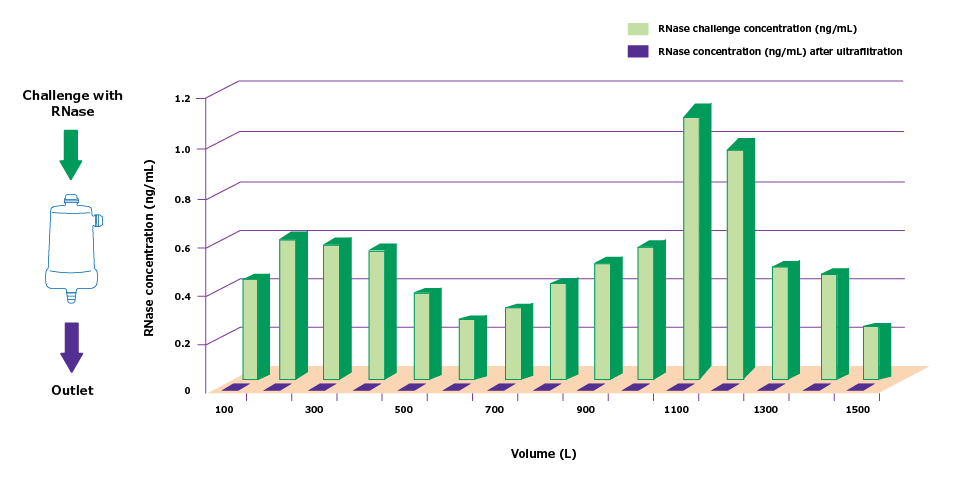
Figure 3. Efficiency of RNase removal from ultrapure water by ultrafiltration using an ultrafiltration cartridge.
Advantages of ultrafiltration vs. DEPC treatment
Ultrafiltration is an alternative to DEPC treatment to obtain RNase-free ultrapure water from a water purification system. It presents several advantages versus DEPC treatment:
Convenience and speed: Nuclease-free water is delivered on demand by the water purification system equipped with an ultrafiltration cartridge.
Sustainability: Nuclease-free water is produced in-situ; no chemical treatment, autoclaving or bottle transportation is required.
Water quality: The nuclease-free water is freshly purified and can be used immediately. In addition, since no chemical additive is used, the water quality is maintained.
Safety: No toxic chemicals are used.
Lab Water Solutions for Molecular Biology Research
Freshly purified ultrapure water that has been ultrafiltered at the point of dispense is recommended for studies requiring nuclease-free water. Milli-Q® ultrapure water purification systems can be fitted with a Biopak® point-of-use ultrafilter that comes with a certificate of quality guaranteeing RNase-, DNase- and bacteria-free water for 90 days.
Related Products
Lab Water Solutions Support
If you would like support to determine the optimal water purification solution for your laboratory and its applications, please complete and submit the form below. Please specify your interest so we can customize our follow-up according to your needs. Fields indicated with an asterisk are required.
References
To continue reading please sign in or create an account.
Don't Have An Account?