Membrane Filters for Microplastics Analysis
For easy navigation, click on the links below:
What are Microplastics?
Microplastics are small plastic particles with a size range between 1 and 5 millimeters that have become a significant environmental concern due to their potential impact on marine and freshwater ecosystems and human health. Discoveries related to microplastics in the environment have multiplied at an astounding pace in recent years. These diverse particles have entered the environment at a staggering rate from a variety of sources such as water bottles, packaging materials, clothing, industrial waste, tires, personal care products, and cleaning products.
Microplastics are categorized as being of “primary” or “secondary” origin. Primary microplastics are those that are intentionally manufactured in their size and shape for a broad variety of applications, such as microbeads used in personal care products and production pellets for injection molding. Secondary microplastics result from the degradation of larger manufactured plastic items in the environment such as plastic bags, water bottles, and packaging materials.1 Mechanical abrasion, ultraviolet (UV) radiation, (micro)biological degradation, and general wear and tear of plastics lead to the formation of microplastics, most commonly polypropylene (PP), polyvinylchloride (PVC), polyurethane (PUR), polyethylene terephthalate (PET), and polystyrene (PS). These particles are pervasive environmental pollutants found in oceans, rivers, lakes, and even in the air we breathe.
Microplastic Regulations
The regulation of microplastics varies widely across different parts of the world, with some countries and regions taking a more proactive approach than others. In many developing countries where waste management systems may be inadequate, there is little to no regulation in place to address the release of microplastics into the environment. In other countries, in-house analytical methods have been developed due to the immediate need for testing.
Europe
The European Union (EU) has taken a leading role in regulating microplastics. In January 2019, the EU adopted a ban on the use of microplastics in several products, including microbeads in personal care products. The ban prohibits the manufacture and sale of products with intentionally added microplastics, with some exceptions.2 The EU also set limits on the amount of microplastics that can be released into the environment, requiring member states to monitor and report on the levels of microplastics in the environment.
North America
Currently, there are no specific federal regulations on microplastics in the United States. However, there are laws and regulations that indirectly address microplastics, and some states have taken their own actions. For example, in 2015, the Microbead-Free Waters Act was signed into law, which banned the use of plastic microbeads in rinse-off cosmetic products.3 The Clean Water Act regulates the discharge of pollutants, including microplastics, into the nation’s waters.4 Several states, including California and New York, have introduced legislation to ban or restrict the use of microplastics in certain products, such as personal care products, single use plastic bags, and cleaning products.
In Canada, policies have been and are being developed that target specific subsets of microplastics. These include a ban on the sale and manufacture of microbeads in toiletries (2019) and on six single-use plastic items that are common sources of secondary microplastics (2022).5
Asia
Several countries in Asia have taken steps to regulate microplastics. For example, in China, there is a ban on the production and sale of microbeads in personal care products.6 Japan has also implemented a voluntary program in which companies can commit to reducing the use of microbeads in their products.7 In South Korea, there is a limit set on the size of microplastics that can be used in personal care products.8 Both Australia and New Zealand have banned the use of plastic microbeads in personal care products. In addition, the Australian government has launched a National Plastics Plan, which includes measures to address microplastics in the environment.9
Standards
ASTM and ISO standards organizations have focused recent efforts on microplastics methods, specifically around sampling.10-11 ASTM also has several active working items to develop new methods for analytical detection of microplastics in environmental waters. ISO has a similar draft related to microplastics analysis which pertains specifically to microplastics from textile sources (e.g., ISO/DIS 4484-2.2).12 Development of methods has proven challenging for many reasons, including the variety of analysis techniques and considerable sample diversity. While there are some regulations and legislation on microplastics in various parts of the world, there is a need for greater global cooperation and coordination to address this pervasive pollutant.
In the United States, two published standards for analysis are important to note. In 2015, the National Oceanic and Atmospheric Administration (NOAA) published a methods manual for measuring microplastic in the environment, which involves (1) sieving and drying, (2) organic matter elimination (oxidation step), (3) density separation, (4) microscopic examination, and (5) gravimetric analysis.13 In 2022, California’s State Water Resources Control Board (SWRCB) approved the world’s first standardized methods for testing microplastics in drinking water. Two standard operating procedures (SOP) were developed and validated through a multi-laboratory study: analysis by infrared spectroscopy and Raman spectroscopy.14
Workflow for Analyzing Microplastics
Currently, there is no universal standard method for analyzing microplastics, making it difficult to compare results from different studies. Various approaches for the quantification and identification of microplastics have been published and utilized largely by academic and government research institutions as well as independent testing laboratories across the globe. Many methodologies are based on previously published reports from NOAA and SWRCB.13,15 While these publications cover a wide variety of matrices, from environmental water samples and seawater to drinking water, animal tissues, food, and air, almost all methods follow the similar general workflow involving sample collection, sample preparation, filtration, visual identification, spectral or chromatographic analysis, and data analysis and reporting.16
Sample Collection for Microplastics Analysis
The sampling method strongly depends on the goal(s) of the research, sampling location, and the type of microplastics being studied. Proper techniques must be used to prevent contamination. For water and sediment samples, sampling methods may include the use of nets, filters, and sediment traps. Despite the abundance of research and publications on microplastics, collection is the only portion of the workflow for which there are published standard practices. Further best practices include adequate water volume, minimization of contamination, and the use of positive controls.17
In 2020, ASTM D8332-20 and ASTM D8333-20 methods were released that outline sample collection protocols for identification and quantification of microplastics and preparation for other analytical methods [infrared (FTIR) spectroscopy, Raman spectroscopy, or gas chromatography coupled to mass spectrometry (GC-MS/MS)].10,11,18 ASTM Method 67563 (also in development) provides guidance on the collection of municipal sewage and treated wastewater effluent for microplastics analysis. The International Organization for Standardization (ISO) has two standards under development related to sampling microplastics in water: (1) ISO/CD 5667-27.2, Part 27 describes the procedure for collecting suspended microplastic particles in different water matrices such as drinking water, surface waters, freshwater, seawater, wastewater-treated effluents, and untreated wastewater, and (2) ISO/CD 16094-1, which lays out the general principles for microplastics analysis and provides guidance on sampling of plastics in waters with low contents of total suspended solids (TSS) such as drinking water, ground water, precipitation water, surface water, and water resulting from water treatment processes.19,20
Sample Preparation for Microplastics Analysis
Once samples are collected, they must be processed and prepared for analysis. Except for clean samples like drinking water and bottled water, samples usually have a high load of organic and inorganic contaminants and particulate matter other than the plastic particles under investigation. Thus, samples should be clarified, enriched, and/or concentrated before analysis. In a multi-lab study conducted by SWRCB,14 multiple sample preparation methods were utilized as there are no regulations to date that provide guidance for this step.
Sample preparation methods can be grouped into three categories:21
- Physical – these methods are used for sediment samples to reduce inorganic content and concentrate the microplastics. The most common physical method is density separation, which is based on the differences in density between the microplastic and sediment particles. Density separation is usually achieved by agitating the sample in concentrated salt solutions such as sodium chloride (NaCl), calcium chloride (CaCl2), sodium iodide (NaI), zinc chloride (ZnCl2), or potassium carbonate (K2CO3).22
- Chemical/Wet Oxidation – these methods are used for organic-rich samples (such as wastewater and biota). While there are a variety of different strategies reported, common chemical reactions used for oxidation include ~30% (v/v) hydrogen peroxide (H2O2) solution in combination with iron salts as a catalyst (Fenton reaction) or in combination with sulfuric acid (H2SO4). Concentrated strong bases (sodium or potassium hydroxide, NaOH, KOH) and concentrated oxidizing acid solutions (HNO3, HNO3/HClO4) have also been used.23 In this step, the chemical compatibility of any consumables that come into contact with the solution should be considered.
- Enzymatic – these methods are used when a “gentler” digestion method is desired, such as when analyzing sensitive synthetic polymers that could be lost or degraded during chemical digestion.24
The optimal sample preparation method depends on the complexity of the matrix and typically requires a combination of extraction techniques designed to isolate the microplastics from the sample matrix.
Production of Microplastics Analysis Grade (MAG) Water
In California’s Policy Handbook, microplastics analysis grade (MAG) water is to be used as reagent water and to rinse all labware before use. MAG water is defined as “high purity water filtered through a filter with pore-size 1 μm or smaller (of any appropriate material; glass fiber filters are suitable).”14 Table 1 shows different types of Millipore® membranes having pore size of 1 μm or smaller and their efficiency in retaining polystyrene beads of various diameters (to mimic microplastics). It is recommended that those membranes with retention of 1.0 µm and lower should be used to prepare MAG water; however, some depth filters and those with pore sizes close to 1.0 µm, such as 1.2 µm MCE, can also sufficiently clear particles of 0.96 µm diameter.
Table 1. The average percent retention (mean ± STDEV, n=3 discs) of polystyrene beads diameters 5.09, 0.96, 0.784 µm, by Millipore® membrane filters of varying materials and pore sizes.
Sample Filtration for Microplastics Analysis
Filtration involves passing the sample through a setup with a filter holder and membrane filter that traps the microplastics, which can then be isolated and analyzed. The type of filter used in a method can impact experimental results.
Choosing the right filter for collecting microplastics is not as simple as it may first appear. While many filters can successfully collect microplastics, the impact of the filter material on the success of any downstream analysis should be carefully considered. However, those looking to collect microplastics on a filter surface should generally consider the following:
- Pore size and retention: Microfilters typically greater than 0.2 µm pore size are suitable for microplastic analysis. However, if specific size ranges of microplastics are desired (for example, 1 µm particles or larger), larger pore sizes may be more appropriate. Microfilters range from 0.2 µm to 100 µm pore size.
- Polymer material: While most filter materials are compatible with aqueous samples, those samples that must be prepared using digestion methods or density separation will require a filter that is chemically compatible with the bulk reagent in which the sample is filtered. Further, consideration of membrane wrinkling, curling, or other morphological changes after the filter is wetted and/or oven-dried prior to analysis should be considered. Lastly, some membrane filters are made of the same components as plastics that might be of interest. Consider filters that are free of particulates or fibers.
- Filter diameter: Small diameter filters allow for a smaller surface to analyze, decreasing analysis time. However, the smaller the filter, the lower the volume that can be investigated. In general, larger filters are appropriate for larger volume samples while smaller filters appropriate for smaller volume samples. Filtration of samples containing higher particulates may be more efficient with larger diameter membrane filters due to increased surface area and a lower chance of clogging.
- Handleability: Thinner filters such as polycarbonate can be difficult to handle using forceps, especially when wetted. If the filter needs to be removed (physically moved from the filtration setup to some other analysis setup), thicker and sturdier membrane materials may be needed to avoid incidental folding and wrinkling. For some membranes, low handleability can lead to low particle recoveries due to rolling and/or electrostatic movement of particles off the filter surface during transport. This can also be caused by smoother surfaces.
Filtration equipment
The most common setup for filtration is shown in Figure 1. The membrane filter is held in place within a filter holder that is attached to a flask; a clamp secures the setup. There are a few considerations when choosing a filter holder: the choice depends on the sample and type of membrane filter to be used.
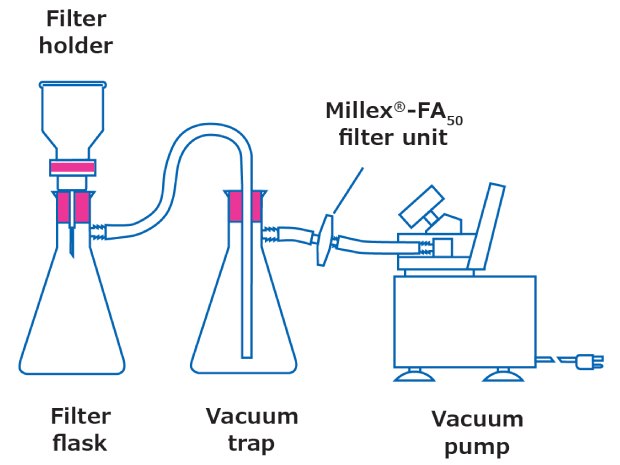
Figure 1.Filtration setup
- Filter holder material: Typically, glass, stainless steel, and plastic filter holders are available. Stainless steel filter holders have broadest chemical compatibility. Hydrosol™ stainless steel filter holders can be grounded and thus used for the safe filtration of volatile solvents. Glass setups are the easiest and most versatile setups, while plastic filter holders are designed for one-time use and disposal.
- Diameter and effective filtration area: The diameter of the filter not only dictates the filtration area that the sample can be dispersed upon but also the sample volume that can be handled. The right filter size can make filtration easier. In some cases, the filter holder may have a glass frit with a PTFE seal. Those wishing to avoid plastic components should ensure their filtration setup is entirely made of glass. The effective filtration area (EFA) of the membrane exposed to the sample varies by filter diameter and filter holder setup. Often, EFA is provided in product literature.
- Filter support: The support (base where the filter sits) can be multiple materials, including a glass frit or a stainless-steel mesh. In some cases, a PTFE gasket is included on the outside for additional stability. To avoid plastic components in your filtration setup, you should ensure that the filter holder is entirely made of glass or stainless steel.
- Non-specific binding: It is possible for microplastics to adhere to filtration setups. In such a case, additional rinses with MAG water can be implemented both when the funnel is clamped and when gently lifted off the filter with vacuum still applied.
Visual Identification and Spectral or Chromatographic Quantitation
A variety of methods are used alone or in combination to accurately quantify microplastics in environmental matrices (Table 2). Many early studies utilized light microscopy to identify microplastics in samples. In this workflow, particles were identified based on color and shape. Fluorescent methods have been adapted and are more commonly used.25 Electron microscopy can be used for detailed surface characterization. Over the past few years, more advanced techniques have been developed to be used in tandem with microscopic methods.26 Spectroscopy, such as Fourier transform infrared spectroscopy (FTIR), Raman, and variations on these techniques can determine the chemical structure of specific polymer (plastic) types and are non-destructive. Pyrolysis combined with gas chromatography mass spectrometry (Py-GC/MS) can give very specific information about particle composition and even the additives in those particles, but is a destructive method of analysis. In all these cases, a filter membrane is typically used as the substrate for the particles after collection. Different filters are better for certain methods over others, as discussed in the next section.
Table 2. Methods used to analyze microplastics.
Data Analysis and Reporting
The data collected during microplastics analysis needs to be analyzed and interpreted to be presented in an insightful way. This may involve statistical analysis, mapping, and other visualization techniques. Typically, it is of interest to utilize data to learn:
- Polymer types and composition
- Size and shape information
- Particle concentration
Filter Considerations for Microplastics Analysis
Filtration plays a key role in the analysis of microplastics. Filters come in a wide variety of membrane materials, pore sizes, and diameters to accommodate the different requirements of analytical methods. In many microplastics workflows, the filter membrane not only collects the particles, but also acts as a substrate on which they are characterized. Filters as a substrate must be flawless, flat, allow optical particle recognition, and must not interfere with the analysis, in addition to having the right pore size to retain the particles during collection.27
Filter Considerations for Microscopy and Optical-Based Methods in Microplastics Analysis
- Fluorescent interference
- Optical clarity
- Filter material refractive index
- Contrast vs. microplastic particles
- Surface roughness or smoothness
- For particle retention
- For well-focused images
- Changes of surface structure upon wetting/drying
- Presence of fibers
- Gridded vs. non-gridded
- Handleability from filtration setup to microscope
- Particle retention
Visualization and optical-based analytical methods typically employ glass fiber membrane filters. For light microscopy studies, a flat, smooth, and gridded membrane filter such as optically transparent polycarbonate or optically white MCE may be important for visualization and counting. However, in cases where plastic particles are smooth, a rougher, thicker, sturdier membrane surface such as polypropylene, quartz fiber, or glass fiber can help embed particles. Some studies have used Nile Red to detect and quantify microplastics because of the dye’s selective adsorption onto microplastics and fluorescent properties.25 In this workflow, it is important that the filter membrane does not exhibit fluorescent interference or non-specifically absorb Nile Red. For glass fiber filters specifically, one must determine whether the presence of fibers on the surface of the membrane interferes with analysis of fiber particles.
We have shown that our Millipore® 1.0 µm glass fiber membrane filter is suitable for use in Nile Red methods to quantify plastic particles in drinking water (Figure 2-3) using an adapted method.25

A) Millipore® 1.0 µm glass fiber filter, replicate 1

B) Millipore® 1.0 µm glass fiber filter, replicate 2

C) Millipore® 1.0 µm glass fiber filter, replicate 3

D) Control 1.5 µm glass fiber filter
Figure 2. Representative images of microplastics under blue light using Nile Red fluorescent stain collected on a Millipore® 1.0 µm glass fiber membrane (replicates 1-3 in A-C, respectively) compared to those collected with the 1.5 µm glass fiber membrane (D) suggested in a published method.25
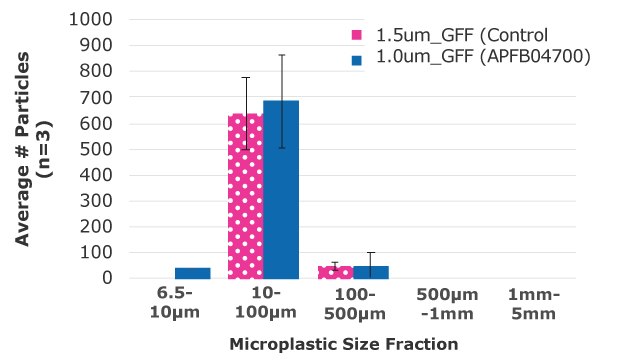
Figure 3.The average number of particles was determined within five size fractions of microplastics between 6.5 µm-1 mm diameter on each membrane.
A variety of other membranes were investigated for contrast and optical clarity while imaging model microplastics. It was found white glass fiber, quartz fiber, both white and clear polycarbonate (PC), black mixed cellulose ester (MCE), and white MCE filter membranes showed good retention of particles in their respective size ranges (see Table 1) while also displaying quality in imaging (Figure 4 and Figure 5). Glass fiber and quartz fiber filters have rough surfaces that were good for capturing and retaining microparticles during transport, while PC and MCE showed smooth surfaces that were good for characterizing particles efficiently. While glass fiber and quartz fiber filters did not show visual differences when air-dried or oven-dried (65°C) after wetting, black MCE became darker while PC showed watermarks after drying. Achieving the correct contrast and lighting at low magnifications (Figure 4) was difficult using PC due to its shiny surface, making it ultimately more suited to electron microscopy conditions or higher magnifications (Figure 5).

Figure 4.Representative images of model microplastics on 1.0 µm glass fiber filters (A), quartz fiber filters (B), 0.8 µm polycarbonate filters (C), 8.0 µm polycarbonate filters (D), 0.8 µm black mixed cellulose ester filters (E), and 1.2 µm mixed cellulose ester filters (F). Rows from left to right indicate neat filter wetted in PBS buffer and air-dried (overnight), filter with microplastics wetted in PBS and air-dried (overnight), neat filter wetted in PBS buffer and oven-dried for 1 hour at 65°C, and filter with microplastics wetted in PBS buffer and oven dried for 1 hour at 65°C. 20x magnification, bar = 107 µm.

Figure 5.Representative images of model microplastics on 1.0 µm glass fiber filters (A), quartz fiber filters (B), 0.8 µm polycarbonate filters (C), 8.0 µm polycarbonate filters (D), 0.8 µm black mixed cellulose ester filters (E), and 1.2 µm mixed cellulose ester filters (F). Rows from left to right indicate neat filter wetted in PBS buffer and air-dried (overnight), filter with microplastics wetted in PBS and air-dried (overnight), neat filter wetted in PBS buffer and oven-dried for 1 hour at 65°C, and filter with microplastics wetted in PBS buffer and oven dried for 1 hour at 65°C. 100x magnification, bar = 100 µm.
Filter Considerations for IR Spectroscopy
- Spectral interference
- Signal masking
- Thickness
- Reflectivity
- Signal enhancement
- Laser compatibility
- Handleability and fragility
- Disc size and instrument compatibility
- Filter dryness
- Particle size as it relates to particle retention
- Filter availability and cost
- Coating reactivity
A range of different filter types has been tested to determine which is best suited for use in the analysis of microplastics by IR. In reflectance measurements, gold-coated polycarbonate membrane has been shown to provide good performance. For transmittance measurements, aluminum oxide and silicon are commonly used membrane materials.27 In the SWRCB method, the foundation method from which new ASTM methods are being drafted, both FTIR and Raman spectroscopy methods suggest the use of polycarbonate membranes.14
A study of FTIR instrumental conditions for the characterization of microplastics found that stainless steel meshes successfully collected microplastics and enabled their analysis via FTIR microscopy with little spectral interference due to their highly reflective nature, while glass fiber and cellulose ester filters showed spectral interferences at various wavenumbers of interest.28 Figure 6 provides an example of ATR-FTIR background spectra for various filter types discussed in this article, including glass and quartz fiber, polycarbonate, and mixed cellulose.
![Background IR Spectra of Membrane Filters Background IR spectra [absorbance (0-1.0)] vs. wavenumber (cm-1) for various filter membranes discussed through this article. A) 1.0 µm glass fiber, B) 0.7 µm glass fiber, C) quartz fiber, D) 0.4 µm black polycarbonate, E) 0.8 µm white polycarbonate, F) 8.0 µm transparent polycarbonate, G) 0.8 µm black gridded mixed cellulose ester, H) 1.2 µm white mixed cellulose ester, I) 0.45 µm hydrophilic PTFE, J) 0.45 µm hydrophilic polypropylene.](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/environmental-testing-and-industrial-hygiene/background-ir-sepectra-membrane-filters/background-ir-sepectra-membrane-filters.jpg)
Figure 6.Background IR spectra [absorbance (0-1.0)] vs. wavenumber (cm-1) for various filter membranes discussed through this article. From top to bottom: 1.0 µm glass fiber, 0.7 µm glass fiber, quartz fiber, 0.4 µm black polycarbonate, 0.8 µm white polycarbonate, 8.0 µm transparent polycarbonate, 0.8 µm black gridded mixed cellulose ester, 1.2 µm white mixed cellulose ester, 0.45 µm hydrophilic PTFE, and 0.45 µm hydrophilic polypropylene. iD7 ATR (Thermo Fisher Scientific), system mode: reflection, ATR, resolution 4 cm-1; 16 scans.
Filter Considerations for Raman Spectroscopy
Filters for Raman analysis should not exhibit any bands in the spectral range of the polymer (microplastic) material that is being studied and should not be fluorescent. Gold-coated polycarbonate, aluminum oxide, silicon, and glass fiber filters have been studied for microplastics analysis by Raman spectroscopy. Gold-coated polycarbonate filters are sold for research purposes but, similar to silver and other coated membranes, are expensive. It is also possible to see polycarbonate peaks through gold. Aluminum oxide filters are relatively less expensive, but display a weak fluorescence profile that could interfere with analysis.29 Silicon filters are rigid and provide good visible images, but silicon peaks are present in spectra. These filters are not widely available. Glass fiber is inexpensive, but the surface is not smooth enough to make particles easily visible. Likewise, it is common to analyze on glass slides, but if size fractionation and sample preparations are needed for a sample, there must be a way to easily subsample or transfer microplastics particles from the filter surface onto the glass slide.
Filter Considerations for GC/MS and Pyrolysis-GC/MS
- Filter diameter
- Ability to fit in pyrolysis cup
- Subsampling
- Sturdiness
- Background
Highly sensitive analyses can be performed on microplastics using GC-MS. Glass fiber and quartz fiber membrane filters are both used in py-GC/MS methods. Because pyrolysis sample preparation uses pyrolysis to break polymers into smaller moieties, pyrolysis can identify not only the polymer monomer but also any additives used in the fabrication for coloring and other added properties. While this is highly quantifiable using a variety of existing libraries on microplastics, the surface used to collect the microplastics - the membrane filter - should demonstrate low and/or highly distinguishable backgrounds from those of the suspected microplastic polymers. The sample must also be small enough to fit into a typical pyrolysis cup (diameters on the millimeter scale). This can be challenging when utilizing glass fiber filters without binder or quartz fiber filters, where 25 mm is typically the smallest disc size available. Glass fiber depth filters used for gravimetric and suspended solids testing (such as AP400100) can work as well as microfilters in many cases, and come in diameters as small as 10 mm. If small diameters are not available, a portion (13 mm or 4 mm) can be punched out from a larger diameter or membrane sheet, or the entire filter can be folded and placed inside the cup. While py-GC/MS is highly accurate, only a small portion of the sample can be analyzed.
Microplastics are emerging contaminants found ubiquitously in the environment, biota, food and beverages, and even human tissues. There are currently no universal standards to collect, process, and analyze samples despite the hundreds of research papers published on this topic. Organizations like ASTM and ISO are developing standard methods to address this need. There is a wide variety of analysis methods for investigating microplastic size, concentration, and type; however, in all cases, a filter membrane is the preferred method of separating different particle sizes and acts as substrate for characterization. Each method involves different considerations when choosing a membrane filter and filter holder. While there are many different approaches to collecting, preparing, and analyzing microplastics in a variety of environmental matrices, the best solutions are likely to be a combination of visual, spectroscopic, and chromatographic methods.
Filter Selection Table for Microplastics Analysis
Other membrane materials, pore sizes, and filter diameters are also available.
Filters for MAG Water
Filters for Visualization
Filters for Spectroscopy
Filters for Pyrolysis
Filter Holders and Accessories
References
如要继续阅读,请登录或创建帐户。
暂无帐户?