Cannabis Potency Testing: Method Dev. & Cost Considerations
Cannabinoid testing is conducted for a variety of reasons, but always to identify and quantitate various cannabinoids in a source tissue or samples such as tinctures, essential oils, or foods. This technical article discusses common parameters of potency testing that can be problematic to method robustness and presents a method that addresses these issues while offering cost-savings over most HPLC methods currently employed in testing laboratories.
Section Overview
Method Requirements of Cannabinoid Analysis
Most current analytical methods employed for routine testing for cannabinoids utilize reversed-phase chromatography (RPC) with UV detection, and as such require chromatographic separation of the analytes. As the number of analytes to be identified and quantified increases, greater efficiency, and selectivity are required of a method. This often involves fine tuning of the method parameters, such as column chemistry, pH, buffer strength, temperature, and composition of organic modifier.
Mobile Phase pH
At least two parameters that can be problematic are pH and temperature. Fundamental good practice for RPC requires that the pH of a mobile phase should be well-removed from the pKa of the analytes. If the pH is too close to the analyte pKa, there can be large shifts in the retention of the analyte with only a small change in pH. If the mobile phase is not buffered well, then there can be very significant local pH shifts within the migrating analyte band. Therefore, mobile phase pH should be at least 1 pH unit removed from the closest analyte pKa.
In the case of cannabinoids, the analyte pKas are generally between pH 3.5 and 4.0. Unfortunately, this is also the approximate pH of dilute formic acid/ammonium formate mobile phases commonly employed for cannabinoid analysis. However, it is at this pH that resolution of critical pairs is observed despite efforts at method development to keep the pH substantially lower or higher, in order to better control the ionization state of the analytes. In terms of good RPC practice for the development of robust methods, this is necessary for resolution but clearly not ideal.
Column Temperature
It is common to see optimized methods that indicate temperatures of 30 °C or less where the column oven has an integrated cooling unit. Many HPLC column ovens, especially lower-cost units, are configured only with a heating element. Therefore, any attempts to control the temperature at or near room temperature can be problematic. When temperature control at or near such values are critical for resolution, this can result in retention variation if the column oven has no cooling unit and cannot maintain a constant temperature.
Organic Modifier
HPLC methods for the RPC resolution of multiple cannabinoids frequently employ acetonitrile as the organic modifier as a simple solution for the resolution of all sample components. However, a disadvantage with the use of acetonitrile, compared to the other common RPC modifier methanol, is cost. Acetonitrile can cost 3 times as much or more.
Low-cost Methanol Based Gradient HPLC Method
Here we present an alternate method for the analysis of up to 17 cannabinoids that has the following attributes:
- The mobile phase pH is adequately removed from the analyte pKas (mobile phase A pH is ~2.6)
- A higher column temperature is used that can be readily controlled by lower-cost column ovens with heating-only capability
- The organic modifier is methanol, thus potentially lowering costs considerably
- The associated backpressures are such that the method does not require the use of UHPLC instrumentation
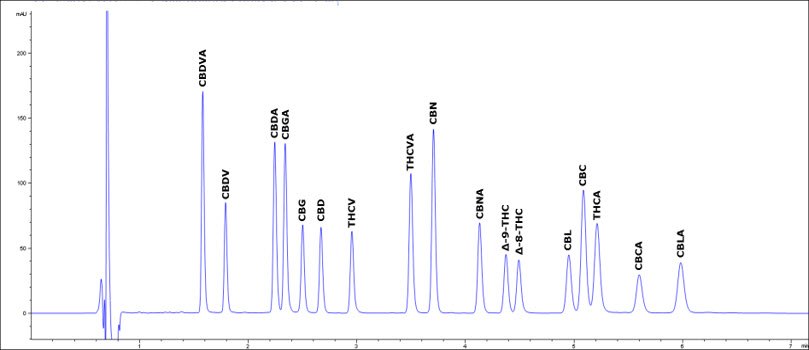
Figure 1.Chromatogram of a typical acetonitrile-based gradient method for potency testing
*CBD recovery not quantitated due to high incurred levels
**Δ9-THC recovery not quantitated due to high incurred levels
ŦCBDV, CBG, CBD, CBC incurred in matrix led to issues preventing calculation of MRL for these compounds
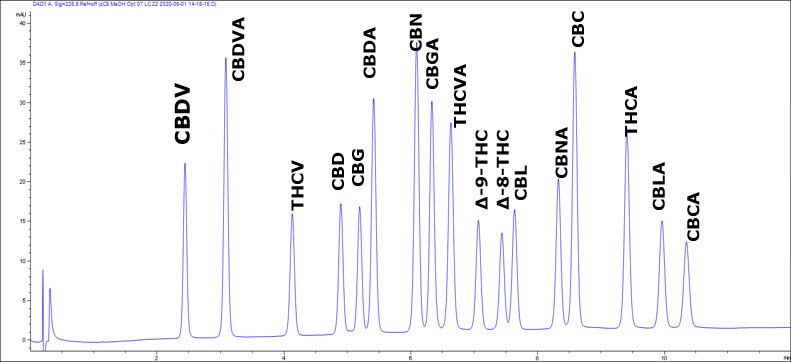
Figure 2.Chromatogram of a low-cost methanol-based gradient method for potency testing
*CBD recovery not quantitated due to high incurred levels
**Δ9-THC recovery not quantitated due to high incurred levels
ŦCBDV, CBG, CBD, CBC incurred in matrix led to issues preventing calculation of MRL for these compounds
Summary
In Figure 1 you see a common method based on the use of acetonitrile as the organic modifier. Figure 2 is the alternate methanol-based method. Experimental conditions are shown in Tables 1 and 2 respectively. While the runtime of the latter is increased 70%, it’s still a very reasonable length, under 12 min. Even with the added length, the cost calculations (Table 3) of the volume of organic solvent consumed is about 3-fold less. As long as the throughput is sufficient, this represents cost-savings that can be readily realized. For either method, further gains in resolution can be attained by using the smaller particle 2 µm Ascentis® Express columns.
Accessories
Certified Reference Materials
Water, Solvents and Chemicals
如要继续阅读,请登录或创建帐户。
暂无帐户?