Pd EnCat™ Encapsulated Palladium Catalysts
We have partnered with Avecia to distribute Pd EnCat™ encapsulated palladium catalysts worldwide.
Homogeneous palladium catalysts are widely utilized due to their versatility, reactivity and functional group tolerance. However, using homogeneous catalysts presents some problems including:
- Probable palladium contamination of product necessitating additional clean up
- Palladium contamination of process equipment with associated decontamination costs
- Heavy metal contamination of waste streams from extraction and washing solvents
- Cost of catalyst lost during reaction and reaction work-up
Pd EnCat™ addresses these issues by using microencapsulation technology to immobilize the palladium, optionally with activating ligands, within a highly crosslinked polyurea matrix.
How it’s Made

Microencapsulation of Palladium (II) Salts by in situ Interfacial Polymerization

Advantages Of Pd EnCat™ Catalysts
- Low residual metal levels in final crude product (typically <10ppm before purification)
- Easy recovery of catalyst by filtration
- Safer and easier to handle than palladium on carbon
- Compatibility with a wide range of process technology options e.g. fixed bed, fluidized bed, trickle bed and microwave reactors
- Efficiency and economy gains through recovery and recycling
- No plating out of palladium on vessel walls
Pd EnCat™ Applications
The performance of Pd EnCat™ is well-documented in an array of widely used transformations. The applications range from C-C bond forming processes, including Suzuki, Heck, Carbonylation, Sonogashira, and Stille coupling, to reductions of carbonyls, alkenes, nitro groups, and epoxides1-9. Selected examples are shown in Tables 1-7. Pd EnCat™ are available optionally with co-encapsulated ligands (Table 2) which simplifies removal of not only Pd but also ligand. Remarkably, these immobilized catalysts mediate high yield transformations often without significant increase in reaction times.
Pd EncatTM40 Catalyzed Suzuki Couplings

Table 1.Suzuki Coupling

Table 2.Suzuki Coupling with Co-encapsulated Phosphines

Table 3.Heck Reaction

Table 4.Carbonylation
Transfer hydrogenations eliminate the need for an external source of hydrogen gas and are readily carried out using nanoparticulate encapsulated Pd(0), Pd(0) EnCatTM 30NP1,6. This unique catalyst is significantly easier to use and safer to handle than activated palladium on carbon. Table 5 illustrates the mild conditions, high yields, and chemoselectivities achieved in the reduction of p-nitroacetophenone and benzylic epoxides. As with other Pd EnCatTM, Pd(0) EnCatTM 30NP is highly recyclable without significant loss of activity as shown (Table 6). Alternatively, chemoselective hydrogenations may be carried out by pre-activation of Pd EnCatTM to give Pd(0) EnCatTM, yielding an immobilized palladium zero catalyst which facilitates high yields and excellent chemoselectivities in a variety of reductions (Table 7)2.

Table 5.Transfer Hydrogenation with Pd(0) EnCat™30 NP

Table 6.Recyclability
Hydrogenations with Pd(0) EnCatTM40

Table 7.Hydrogenation
Resistance to leaching Pd is a key advantage of the Pd EnCat™ products. Levels of Pd found in crude product are dependent on solvent (Table 8), substrate, and reaction conditions. Typically the Pd level in crude product is 10-20ppm prior to any purification. However, levels can be higher or lower and solvent selection is key to achieve minimum leaching; avoiding DMF and DMA where possible. Pd EnCat™ strongly resist swelling in the majority of solvents (Table 9) which is particularly important for scale up applications.

Table 8.Resistance to Leaching
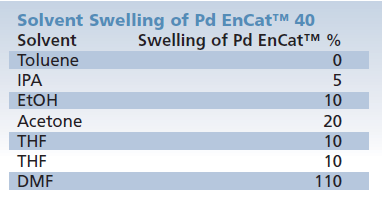
Table 9.Resistance to Swelling
Pd EnCat™ (0.3g, 0.4 mmol/g) was weighed in to a 25mL carousel reaction tube and the solvent (20ml) added. The mixture was then heated to 800C whilst stirring via magnetic stirrer for 2 days. The mixture was allowed to cool to room temperature and filtered through a sintered funnel. The filtrate was collected and analysed for Pd content.
Solvent swell determined by measuring gain in volume of 1g sample of beads in solvent at room temperature over 2h, expressed as %.
Frequently Asked Questions
Are there significant differences between Pd EnCat™ 30 and Pd EnCat™ 40?
While Pd EnCat™ 40 shows excellent performance in a variety of transformations, Pd EnCat™ 30, with lower matrix content, shows similar performance but with improved kinetics while maintaining the mechanically robust nature of Pd EnCat™ 40 under similar conditions. End users are encouraged to screen the sample sets for best fit to application.
What if I would like a different matrix porosity, a different ligand, or a different metal?
Avecia and Sigma-Aldrich intend to collaborate on extensions to the product line. Requests for custom EnCat™ should be directed to Avecia at encat@avecia.com.
Can you provide additional information on using Pd EnCat™?
As a supplement to the referenced literature below, request a free copy of the EnCat™ Users Guide on CD via e-mail at cdavis1@sial.com.
Available Pd Encat™ Products
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?