BioSPME for Plasma Protein Binding Assay: Details of Method Development and Optimization
BioSPME devices coated with C18 adsorbent were successfully used for multiple drug compounds to determine their protein binding properties.1 The methods developed to demonstrate the effect of different extraction volumes and desorption volumes across a range of compounds are described in this document.
A generic method for using a BioSPME C18 pin device with 200 µL sample volume is presented in Figure 1. The extraction is performed from both buffer and plasma samples and protein binding is calculated using the determined extracted amounts.
Outlined below are the calculations for %protein binding:
Equation 1.
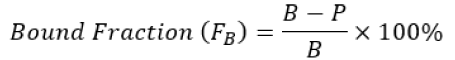
Where
Equation 2.
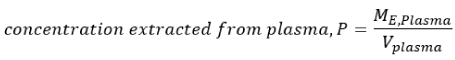
Equation 3.

and ME represents the quantity/amount extracted from the corresponding solution.
Experimental Method
Conditioning - Activation of BioSPME Phase
During BioSPME method development, it is important to condition the C18 coating of the pin device using an organic solvent. Under generic methodology, the conditioning step is done statically, under no agitation. If the coating is not fully conditioned, the extracted amount of analyte can significantly vary between samples decreasing the reproducibility of the extraction method. A short water wash is employed after the conditioning step to remove most of the organic solvent from the coating prior to its exposure to biological matrices to prevent the immediate precipitation of proteins.
Extraction of Analyte with BioSPME
It is recommended that BioSPME extraction step is performed under agitation at 1200 rpm using a shaker with 3 mm orbital radius to provide efficient agitation and faster extraction. It is important to allow enough time for the analyte to achieve equilibrium between the sample solution and the C18 coating. Therefore, the duration of the extraction step is governed by the extraction kinetics and by the type of analyte. It was found in most cases that 15 minutes for the extraction time (in the recommended generic protocol) was sufficient to get close to equilibrium for most analytes in both buffer and plasma (Figure 2) using SupelTM BioSPME C18 devices.
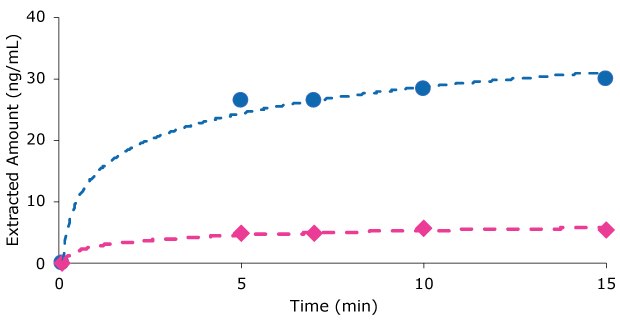
Figure 2. Extracted amount versus time for carbamazepine using Supel™ BioSPME C18 pin device, 200 µL extraction volume, 1200 rpm agitation with 3 mm orbital radius and Hamilton robotics heater/shaker (blue dots) buffer solution or (pink diamonds) plasma samples containing carbamazepine.
Using sample volumes smaller than 200 µL is possible with BioSPME devices. For 100µL volume samples, extraction time could be decreased to 6-10 minutes for analytes as the more efficient agitation at the smaller volumes provides faster equilibrium (Table 1). For sample volumes smaller than 100 µL it is recommended to dilute samples with buffer.
Table 1. Results of protein binding measurements using BioSPME C18 devices and 100 µL samples under agitation. 1 Carbamazepine, 2 Prednisolone, 3 Zolpidem, 4 Warfarin. PB – protein binding
Static extraction (without agitation) is strongly NOT recommended as the equilibration times can increase significantly beyond the reasonable expectation of length of the extraction method. As shown in Figure 3, static extraction of carbamazepine in 60 minutes failed to reach the 15-minute extracted levels of the agitation method.

Figure 3. Static extraction of carbamazepine from 200 µL volume at 100 ng/mL total spiking amount. For comparison, one 15-minute time point is shown for extraction from buffer using agitation (blue dot).
Bio-compatible coatings prevent co-extraction of larger protein molecules from samples. A 60-second wash step carried out statically after extraction further removes non-specifically bound proteins from the coating before desorption.
Desorption - Analyte transfer into Solvent
Desorption of the analytes can be performed statically using a mixture of 80/20% methanol/water for most drugs. It is important that complete desorption of the analyte from the BioSPME coating into the desorption solvent is achieved. A second desorption was performed, in addition to the first, to check that the desorption step was efficient enough to allow complete desorption to occur. The second desorption was carried out using the same freshly prepared desorption solvent and time. The organic modifier in the desorption solvent can be varied with regard to its amount and type depending on the hydrophobicity of the analytes. For example, desorption using acetonitrile can be used for more hydrophobic analytes. In our work, 80/20% methanol/water desorption solvent performed well for all analytes under study with the SupelTM BioSPME C18 pin devices.
Results & Discussion - BioSPME vs. Rapid Equilibrium Dialysis
The analytes and their measured protein binding values are listed in Table 2. In comparison, the values for rapid equilibrium dialysis protein binding were also determined and are shown in Figure 3. A good correlation between protein biding from the two different methods was found.
Table 2. Compounds under study using BioSPME and their protein binding values using the generic BioSPME method and 200 µL samples spiked at clinically relevant concentrations.
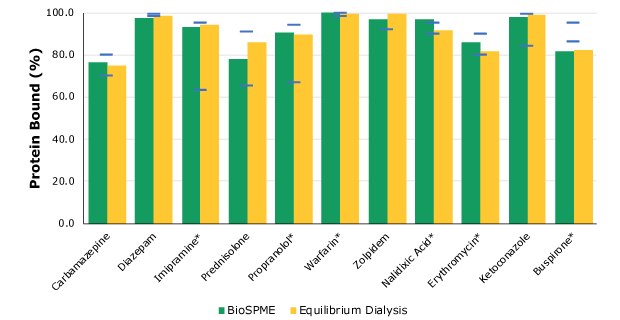
Figure 4. Comparison of protein binding values between BioSPME, solid phase microextraction, (in green) and rapid equilibrium dialysis (in yellow) methods. The blue lines indicate the protein binding literature value intervals. Compounds with stars are charged at physiological pH.
The same extraction method was applied to all analytes. Modification to the method had to be undertaken when analyzing imipramine, propranolol, and ketoconazole. This modification included the use of the glass-coated well plates instead of standard polypropylene 96-well plates due to the non-specific binding to the plastic that imipramine, propranolol, and ketoconazole exhibited from buffer solutions. When non-specific binding is observed, extraction efficiency from buffer using BioSPME decreased for such compounds, and, therefore, the protein binding value could not be correctly calculated. The use of glass-coated plates was also tried and was beneficial for the extraction of erythromycin and buspirone from buffer.
For warfarin, the method modification included the use of 50 µL desorption volumes rather than 200 µL desorption solvent. Warfarin is very highly bound to proteins (98.1-99.6% bound) and is present at a very low free concentration. In order to increase the detection sensitivity desorption into 50 µL volumes was undertaken. It was found that a decrease in the desorption time to 5 minutes was sufficient to achieve efficient desorption and also minimized the evaporation of solvent amount during the desorption step. In general, the prepared desorption plate was covered until used in order to avoid evaporation of the solvent before the desorption step.
The generic extraction method performed well across the range of analytes that had logP values of 1.6-5, were neutral, positively or negatively charged, and had a molecular weight in the range of 236-734 Da.
We have attempted to investigate a few compounds with molecular weights higher than 1 kDa. These included lanreotide and cyclosporine. Larger compounds can carry multiple charges, such as the case with lanreotide, at 1096 Da, with +2 charge at physiological pH. This compound could not be extracted from buffer using C18-coated BioSPME devices. A higher positive charge probably prohibited extraction into the hydrophobic adsorbent coating. Larger compounds also can undergo aggregation in buffer solution, such as the case for cyclosporine. Neutral cyclosporine at 1203 Da could not be efficiently extracted from buffer using either plastic or glass-coated 96-well plates. For this compound, the time to equilibration was also found to be longer than 4 hours. Therefore, it is not recommended to use the BioSPME C18 method for compounds larger than 1000 Daltons or compounds that carry multiple charges at physiological pH.
Conclusion: BioSPME Device Helps in Effective Measurement of Protein Binding Properties
A generic method for using SupelTM BioSPME C18 pin devices was successfully applied to determine plasma protein binding properties of several compounds. The compounds were spiked at the same level into both buffer and plasma, extracted using the proposed method, and protein binding was calculated using the extracted amounts. The method optimization steps for different compounds and different sets of conditions are presented. The obtained protein binding values were within the literature range and matched those achieved using equilibrium dialysis.
Troubleshooting BioSPME
1. How can I achieve less than 15% reproducibility on extraction?
Review the following steps in the extraction and detection method to ensure good extraction reproducibility:
- Conditioning of BioSPME is performed as required to activate the adsorbent
- Coating is not allowed to dry between conditioning and extraction and between extraction and desorption steps, transition of the pin tool between the method steps is done within 10-20 seconds
- The levels of solvents and the sample in the wells are high enough to fully submerge the coating
- Pin tool is leveled across the well plate and the grippers work correctly
- The analyte in buffer solution was tested for the non-specific binding to the plastic plates. If non-specific binding is exhibited by analyte, the use of glass-coated plates is recommended
- The pH of the sample is the same across multiple extractions
- The temperature of the samples under extraction is well-controlled using heating adapters; the temperature across the well-plate can be checked using thermocouple when the setup of the method is performed
- The spiked sample is well-equilibrated prior to extraction, we recommend using at least 1 hour incubation time
- The extraction time is sufficiently long to achieve equilibrium but not too long to allow the extraction competition from sample lipids. Too short and too long extraction times can also result in lower accuracy of the protein binding values
- The desorption time is sufficiently long to allow full desorption of the analyte from the coating into the organic solvent
- LC-MS instrument reproducibility is checked and found to be acceptable (at 3-4% CV) when sample in buffer is injected multiple times. LC-MS detection is within lower limit of quantitation
2. How can I adapt the method for my analyte?
Method parameters that can be changed include sample volume, extraction time, volume of desorption solution, desorption time. Reaching equilibrium extraction conditions and full desorption of the analyte will need to be confirmed for method changes.
3. How to mitigate the effects of non-specific binding of compounds to plastic well plates?
Hydrophobic compounds (with logP of 3.5 and above) can undergo non-specific binding to the plastic well plates. Some of the positively charged compounds were also found to have lower extraction efficiencies from plastic polypropylene plates in buffer. This can significantly change the extracted amounts from buffer and result in the introduction of errors into protein binding values found by BioSPME.
Common ways to eliminate the non-specific binding, such as the introduction of surfactants to the analyte solutions, were found not to be acceptable. Any additives can be extracted by BioSPME and contaminate the LC-MS instrumentation. Additives can also change buffer properties and influence extraction equilibrium. It was found that using glass-lined plates resolved the non-specific binding issues for most analytes studied during BioSPME development. These plates are commercially available from NS3inc.com, for example.
See FAQ for more method development tips in our Publications:
Supel™ BioSPME C18 96-Pin Devices User Guide for Automation Workflow
Supel™ BioSPME C18 96-Pin Devices User Guide for Manual (Non-Automation) Workflow
Related Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?