Bioanalysis with SPME
Technical content was generated and provided by:
Solid phase microextraction (SPME) offers rapid sample preparation both in the laboratory and field.1 The basic concept of the technology is a sorbent-coated rod that is put into contact with a sample (gaseous, liquid, semi-solid) or the headspace of liquids or solids. The sorbent is selected to have good affinity for the analyte of interest in the sample. After a pre-defined exposure time, sufficient analyte will have moved from the sample to the sorbent to permit quantitative analysis. The amount extracted is proportional to the original concentration of analyte in the sample, permitting simple determination of sample concentration.
Figure 1 illustrates the basic concept of the commercial SPME device introduced by Supelco in 19931 that has seen wide application in a variety of fields.
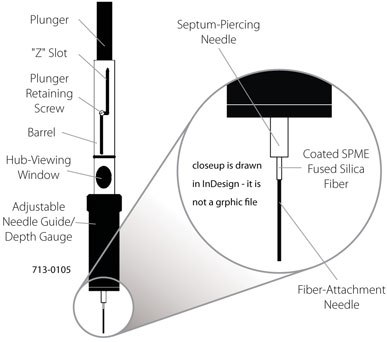
Figure 1.Design and Enlarged View of the First Commercial SPME Device Made by Supelco.
A new line of SPME devices has been recently introduced to better address bioanalysis opportunities
(Figure 2). These devices employ C18 bonded porous silica sorbent particles, similar to particles typically used in HPLC columns or as SPE sorbents, in a proprietary biocompatible binder. The binder used is a non-swelling polymer which resists fouling by biological matrix components. After extraction, solvent desorption is performed in a small volume (50-100 μL) and the desorption solution directly injected, typically to LC or LC-MS. The solid support used for these probes is a flexible metal alloy (0.008”/203 μM diameter), offering both robustness and an inert support, and the coatings are housed inside a 22 gauge hypodermic needle with an incorporated seal to prevent sample wicking. Because of the C18 extraction phase, they behave as an absorptive phase. They may be employed as single use devices and are ideally suited for either in vitro sampling directly from whole blood or plasma in sample vials sealed with hole caps and septa, or through an injection bulb on an intravenous catheter for in vivo analysis. Devices without the attached hypodermic needle are also available where sealing is not critical, e.g. tissue sampling or sampling from open vials. The operating principles are analogous to the conventional SPME devices.
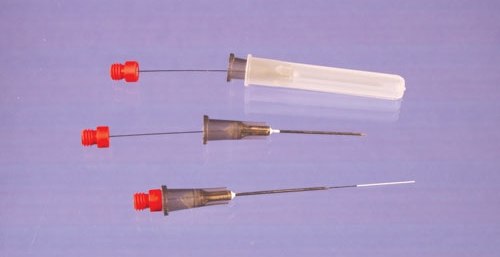
Figure 2.New Biocompatible SPME Devices for Bioanalysis and in vivo Sampling (45 μM thickness, 15 mm length of the coating, Cat. No. 57281-U).
An important advantage of SPME, particularly for on-site sampling, is the possibility of performing analyses without predefining a specific sample size. For SPME from small volumes of sample, the amount of an analyte extracted from a sample is given by Equation 11.
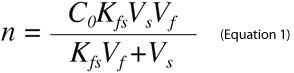
where Co is the initial sample concentration of the analyte, n is the amount of analyte extracted, Vs is the sample volume, Vf is the fiber volume and Kfs is the analyte distribution constant between the fiber and sample matrix. However, when sample size is large relative to the fiber capacity (Vs>>VfKfs), Equation 1 reduces to Equation 2, which renders the amount of analyte extracted by SPME independent of the sample volume.
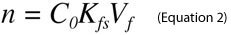
This simplìcation is valid for most on-site analyses and eliminates the need to remove a representative sample from the system under study in order to perform the analysis. From a bioanalytical perspective, this permits the use of SPME to directly sample blood or tissue of animals in vivo, without having to first withdraw a biofluid/tissue sample. New calibration approaches including internal standardization by pre-loading standards on the SPME device allow rapid pre-equilibrium sampling and control of variability in complex matrixes.
Initially, in vivo SPME was used to study the pharmacokinetics (PK) of various drugs in directly from the veins of animals. A specialized interface was developed to permit monitoring of small rodents (mice and rats). More recently, in vivo SPME was successfully applied to fish to study bioaccumulation of pharmaceuticals, pesticides and other environmental pollutants using direct muscle or adipose tissue sampling. A survey of a number of additional sorbents has broadened the range of analyte polarities that can be extracted to include highly polar compounds (logP to -8) and has permitted the application of both in vivo and in vitro SPME for non-targeted metabolomics analysis.2
The use of in vivo SPME offers important advantages over conventional methods, such as simplified sample cleanup, fast stabilization of unstable analytes, elimination of enzymatic degradation after extraction, and reduced ion suppression for mass spectrometry analyses. Furthermore, because both sampling and sample cleanup are combined into one step, the number of sample preparation steps is minimized, reducing the potential for analyte loss or accidental contamination. A recent article in Nature Protocols details the steps involved in performing in vivo SPME for intravenous drug and metabolite monitoring.3
Materials
To continue reading please sign in or create an account.
Don't Have An Account?