Purification or Removal of Glycoproteins and Polysaccharides
Con A Sepharose 4B, Lentil Lectin Sepharose 4B, Capto Lentil Lectin
Glycoproteins and polysaccharides react reversibly, via specific sugar residues, with a group of proteins known as lectins.
As ligands for purification media, lectins are used to isolate and separate glycoproteins, glycolipids, polysaccharides, subcellular particles and cells, and to purify detergent-solubilized cell membrane components. Substances bound to the lectin are resolved by using a gradient of ionic strength or of a competitive binding substance.
Chromatography media screening
To select the optimum lectin for purification, it may be necessary to screen different chromatography media. The ligands, Concanavalin A (Con A) and Lentil Lectin provide a spectrum of parameters for the separation of glycoproteins. Table 3.15 gives their specificity.
Con A is a tetrameric metalloprotein isolated from Canavalia ensiformis (jack bean). Con A binds molecules containing a-D-mannopyranosyl, a-D-glucopyranosyl and sterically related residues. The binding sugar requires the presence of C-3, C-4, and C-5 hydroxyl groups for reaction with Con A. Con A can be used for applications such as:
- Separation and purification of glycoproteins, polysaccharides, and glycolipids.
- Detection of changes in composition of carbohydrate-containing substances, for example, during development.
- Isolation of cell surface glycoproteins from detergent-solubilized membranes.
- Separation of membrane vesicles into “inside out” and “right side out” fractions.
Lentil lectin binds α-D-glucose and α-D-mannose residues and is an affinity ligand used for the purification of glycoproteins including detergent-solubilized membrane glycoproteins, cell surface antigens and viral glycoproteins. Lentil lectin is the hemagglutinin from the common lentil, Lens culinaris. When compared to Con A, it distinguishes less sharply between glucosyl and mannosyl residues and binds simple sugars less strongly. It also retains its binding ability in the presence of 1% sodium deoxycholate. For these reasons Lentil Lectin Sepharose 4B is useful for the purification of detergent-solubilized membrane proteins, giving high capacities and extremely high recoveries.
Chromatography media characteristics
Characteristics of Con A and Lentil Lectin chromatography media are shown in Table 3.16.
1 Short term refers to the pH interval for regeneration, cleaning-in-place, and sanitization procedures. Long term refers to the pH interval over which the medium is stable over a long period of time without adverse effects on its subsequent chromatographic performance.
Purification options
Purification options for Con A and Lentil Lectin chromatography media and prepacked columns are shown in Table 3.17.
1 See Appendix 4 to convert flow velocity (cm/h) to volumetric flow rate (mL/min). Maximum operating flow is calculated from measurement in a packed column with a bed height of 10 cm and i.d. of 5 cm.
2 Supplied in acetate buffer solution (100 mM, pH 6.0) containing 1 M NaCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2, 20% ethanol.
Purification examples
Enrichment of Glycoproteins from Human Plasma
Glycoproteins from human plasma were enriched on a HiTrap® Con A 4B 1 mL column (Figure 3.15A). Analysis by Coomassie stained SDS-PAGE (nonreducing conditions), showed that unfractionated plasma (start material), flowthrough, and wash fractions all had band corresponding to molecular weight of 67 000 (Figure 3.15B). This corresponds to high-abundance, nonglycosylated serum albumin, which was removed from the sample and not detected in the eluate containing the glycoproteins.
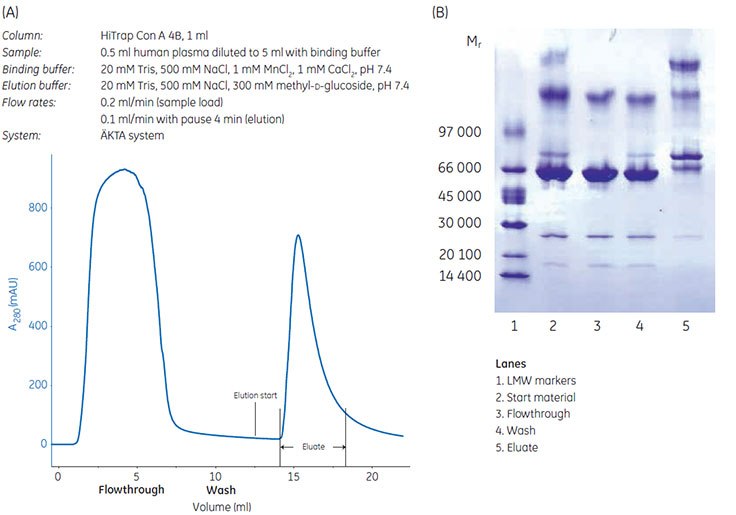
Figure 3.15.(A) Chromatographic enrichment of glycoproteins from human plasma using HiTrap® Con A 4B, 1 mL. (B) SDS-PAGE anaysis with Coomassie stained ExcelGel™ 8–18 Gradient gel (nonreducing conditions) of fractions from enrichment of glycoproteins from human plasma using HiTrap® Con A 4B, 1 mL.
Performing a separation
- Pack the column (see Appendix 3) and wash with at least 10 CV of binding buffer to remove preservative.
- Equilibrate with 10 CV of binding buffer.
- Apply the sample, using a low flow velocity from 15 cm/h, during sample application (flow velocity is the most significant factor to obtain maximum binding).
- Wash with 5 to 10 CV of binding buffer or until no material appears in the eluent (monitored by UV absorption at A280 nm).
- Elute with 5 CV of elution buffer.
Recovery from Con A Sepharose 4B is decreased in the presence of detergents. If the glycoprotein of interest needs the presence of detergent and has affinity for lentil lectin, the Lentil Lectin Sepharose 4B chromatography medium provides a suitable alternative to improve recovery.
For complex samples containing glycoproteins with different affinities for the lectin, a continuous gradient or step elution can improve resolution. Recovery can sometimes be improved by pausing the flow for a few minutes during elution.
Cleaning
Wash with 10 CV of 500 mM NaCl, 20 mM Tris-HCl, pH 8.5, followed by 500 mM NaCl, 20 mM acetate, pH 4.5. Repeat three times before re-equilibrating with binding buffer.
Remove strongly bound substances by:
- washing with 100 mM borate, pH 6.5 at a low flow rate.
- washing with 20% ethanol or up to 50% ethylene glycol.
- washing with 0.1% Tween 20 at 37 °C for 1 min.
Re-equilibrate immediately with 5 CV of binding buffer after any of these wash steps.
Chemical stability
Stable to all commonly used aqueous buffers. Avoid 8 M urea, high concentrations of guanidine hydrochloride, chelating agents such as EDTA, or solutions with pH < 4.0 as these remove the Mn2+ from the lectin or dissociate Con A, resulting in loss of activity.
Storage
Wash chromatography media and columns with 20% ethanol in 100 mM acetate, 1 M NaCl, 1 mM CaCl2, 1 mM MnCl2, 1 mM MgCl2, pH 6.0 (use approximately 5 CV for packed media) and store at 4 °C to 8 °C.
Performing a separation
Buffers for soluble glycoproteins:
Buffers for detergent-solubilized proteins:
Equilibrate column with the buffer 20 mM Tris-HCl, 500 mM NaCl, 1 mM MnCl2, 1 mM CaCl2, pH 7.4, to ensure saturation with Mn2+ and Ca2+.
- Pack the column (see Appendix 3) and wash with at least 10 CV of binding buffer to remove preservative.
- Equilibrate the column with 10 CV of binding buffer.
- Apply the sample, using a low flow velocity from 15 cm/h, during sample application (flow velocity is the most significant factor to obtain maximum binding).
- Wash with 5 to 10 CV of binding buffer or until no material appears in the eluent (monitored by UV absorption at A280 nm).
- Elute with 5 CV of elution buffer using a step or gradient elution.
Below pH 5.0, excess Mn2+ and Ca2+ (1 mM) are essential to preserve binding activity. It is not necessary to include excess Ca2+ or Mn2+ in buffers if conditions that lead to their removal from the coupled lectin can be avoided.
For complex samples containing glycoproteins with different affinities for the lectin, a continuous gradient or multistep elution can improve resolution. Recovery can sometimes be improved by pausing the flow for a few minutes during elution.
Elute tightly bound substances by lowering pH, but not below pH 3.0. In some cases, strongly bound substances can be eluted with detergent, for example 1.0% deoxycholate.
Cleaning
Wash with 10 CV of 500 mM NaCl, 20 mM Tris-HCl, pH 8.5, followed by 500 mM NaCl, 20 mM acetate, pH 4.5. Repeat three times before re-equilibrating with binding buffer.
Remove strongly bound substances by:
- washing with 100 mM borate, pH 6.5 at a low flow rate.
- washing with 20% ethanol or up to 50% ethylene glycol.
- washing with 0.1% Tween 20 at 37 °C for 1 min.
Chemical stability
To avoid loss of activity of the coupled lectin, avoid solutions having a pH below 3.0 or above 10.0, buffers that contain metal chelating agents such as EDTA, high concentrations of guanidine hydrochloride, or high concentrations of urea.
Storage
Wash chromatography media and columns with 20% ethanol (use approximately 5 CV for packed media) and store at 4 °C to 8 °C.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?