Primary Human Hepatocytes Culture Protocol
Primary Human Plateable Hepatocytes Culture Protocol
Section Overview
What are Primary Human Hepatocytes?
Primary human Hepatocytes are cells directly harvested from whole human livers and are used by researchers in vitro to model the physiology and function of hepatocytes. As one of the major cell types of the liver, Hepatocytes function in protein, carbohydrate, and lipid metabolism and help to maintain liver homeostasis1. Because these primary cells can be cryopreserved and then cultured in vitro, they are crucial tools for studying enzyme induction, toxicity, drug screening, transporter efflux activity, potential drug-drug interactions, and disease modeling.
Our collection of cryopreserved human plateable Hepatocytes comprises a homogeneous cell population and is available in diverse pack sizes, including a validated product suitable for use in small volumes within 96-well plates. Each donor provides documented consent for research use of non-transplantable organs or tissues. Every cell lot undergoes extensive characterization, including induction data, enzyme profiling data, and morphological and confluency assessments, with a guaranteed post-thaw viability of ≥70%. This protocol outlines the steps for thawing, plating, and cultivating human plateable Hepatocytes.
Primary Plateable Hepatocyte Culture Materials
- Normal Human Characterized Plateable Hepatocytes (HLP102-3M, HLP102-5M or HLP103-4M)
Note: Upon receipt, immediately store cryovial(s) in vapor phase liquid nitrogen. - Collagen Type I, Rat Tail
- Tissue culture treated multiwell plates
- 1X Hepatocyte Plating Medium (HPM) for Human Plateable Hepatocytes
- 1X Maintenance Medium for Human Plateable Hepatocytes
Note: For Dexamethasone, dissolve 25 milligram into 32 milliLiter of ethanol (100%) to make 2 mM stock. For Linoleic acid, dissolve 100 mg into 2 mL of ethanol (100%) to make 50 mg/mL stock.
Primary Plateable Human Hepatocytes Growth Protocol
All protocols are performed within a Class II laminar flow biohood and with an aspirator unless otherwise specified. Incubators are humidified and are set to 37°C and 5% CO2. PPE should be worn such as gloves, lab coat, and safety glasses.
Preparing a Collagen-Coated Plate
- Dilute the collagen to a final concentration of 56µg/mL in sterile 70% ethanol and gently mix until the collagen is solubilized.
- Add the collagen/ethanol mixture to each well to completely cover the bottom of wells.
- Gently move the cell culture plate until the until the collagen/ethanol mixture evenly coats the inside of the well.
- Air dry plates in a laminar flow hood. Leave cell culture plate over night with the cover ajar to allow airflow and prevent condensation.
Thawing and Plating Primary Plateable Hepatocytes
- Pre-warm a bottle of Hepatocyte Plating Media (HPM) in a 37 ᵒC waterbath for at least 15 minutes prior to thawing the cells.
- Transfer 5 mls of pre-warmed HPM into a sterile conical (15-50 ml); leave the conical uncapped.
- Remove the cryovial and quickly submerge the vial in the 37 ᵒC waterbath.
Note: Do not submerge the cap, only the cells contents portion of the vial. - Allow the vial to thaw for 1.5–2 minutes or until a small spindle of ice is present in the cell suspension.
Note: Do not fully thaw the cell suspension. - Remove the vial from the waterbath and wipe thoroughly with an alcohol wipe.
- Remove the cap and dump the cell suspension into the conical. Rinse the cryovial 2-3 times with warm HPM from the conical to capture any remaining cells.
- Perform a trypan blue count using a 1:5 dilution (50 µL of trypan blue + 350 µL of plating media + 100 µL of cell suspension).
- Adjust the density of the cell suspension as needed using additional HPM.
- Dilute the hepatocyte suspension to the recommended seeding density from the chart below using the HPM.
- Add the diluted cells to collagen-coated tissue culture plates.
- 6-well plate, add 2 mL per well.
- 12-well plate, add 1.0 mL per well.
- 24-well plate, add 0.5 mL per well.
- 48-well plate, add 0.25 mL per well.
- 96-well plate, add 50 µL of HPM per well, then 50µL cell suspension per well.
- Place the plate onto a flat surface and gently shake in a North-South/East-West fashion to evenly disperse the cells.
Note: DO NOT use a circular motion. Observe the well(s) to ensure that the seeding density is appropriate. - Gently place the plates into a 37 °C, 5% CO2 incubator.
- Gently shake the plate(s) every 20 minutes after seeding for 1-2 hours. 6-8 hours after the initial seeding, observe the plate under a microscope at 10X magnification to confirm cell attachment and flattening. The cells should flatten out and begin to form a monolayer with cuboidal shaped cells. If the cells have not flattened out sufficiently, then allow the plate to sit in the incubator overnight.
- Replace the media with 37 °C Hepatocyte Maintenance Medium after cells flatten. If using an overlay like Matrigel®, replace the media with 4 °C Hepatocyte Maintenance Medium + overlay mixture.
- For a 6-well plate, add 1.5 mL per well.
- For a 12-well plate, add 0.8 mL per well.
- For a 24-well plate, add 0.3 mL per well.
- For a 48-well plate, add 0.2 mL per well.
- For a 96-well plate, add 70 µL per well. Note: Do not shake 96-well plates.
- Gently place the plates back into the incubator.
- Replace the Maintenance Medium every 24 hours.
Primary Plateable Hepatocytes Growth Results
Plateable Hepatocytes were plated in HPM overnight on collagen coated plates. Media was changed to hepatocyte maintenance medium before the images were taken on the Millicell® DCI Digital Cell Imager. The plateable Hepatocytes show characteristic cobble stone morphology with well-defined borders.
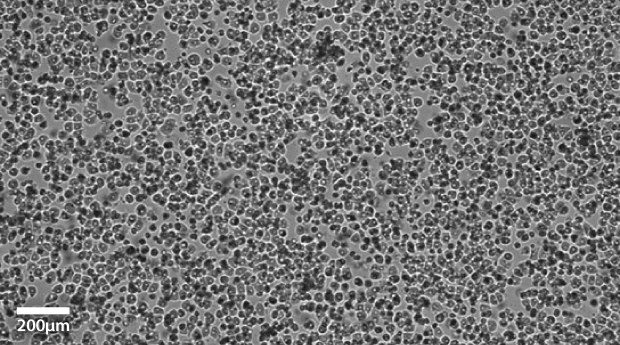
Figure 1A.Primary plateable Hepatocytes imaged after overnight incubation. Imaged with Millicell® DCI Digital Cell Imager at 10x.
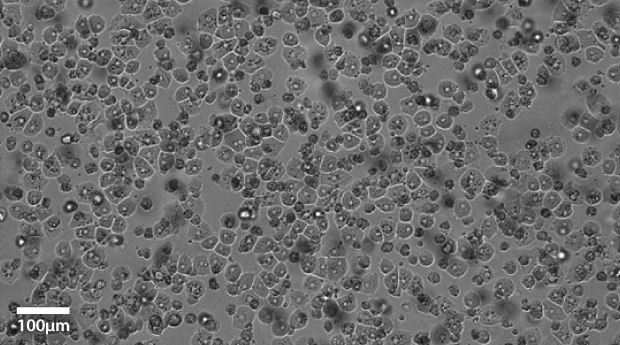
Figure 1B.Primary plateable Hepatocytes imaged after overnight incubation. Imaged at 20x magnification setting.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?