热原测试
热原检测是确保非肠道给药医疗产品(如药品或生物制药)安全的关键步骤。它是强制性释放试验的一部分,以避免热原物质诱发危及生命的发热反应。单核细胞活化测试(MAT)可在一次体外测试中同时检测内毒素和非内毒素热原。
自2025年7月以来,单核细胞活化试验已成为《欧洲药典》规定的检测内毒素和非内毒素的首选方法,取代了兔热原试验(RPT)。
Products
单核细胞活化测试 (MAT)
单核细胞活化测试用于检测药品和医疗器械等肠外产品中的内毒素和非内毒素致热原,提供了一种& in vitro alternative to conventional animal testing in accordance with regulatory guidelines.
兔热原试验和嗜酸性粒细胞裂解液(LAL)试验被广泛用于热原检测。这两种方法都使用动物,有一定的局限性。兔热原试验缺乏稳健性,因为动物的反应可能与人的反应有很大不同。
为了克服这些局限性,《欧洲药典》于 2010 年引入了单核细胞活化试验(Monocyte-Activation Test,MAT)作为药典方法,以取代兔热原试验(EP 第 2.6.30 章)。
请注意: 欧洲药典委员会根据 3R 原则决定终止兔热原试验,认为 MAT 是最佳替代选择。
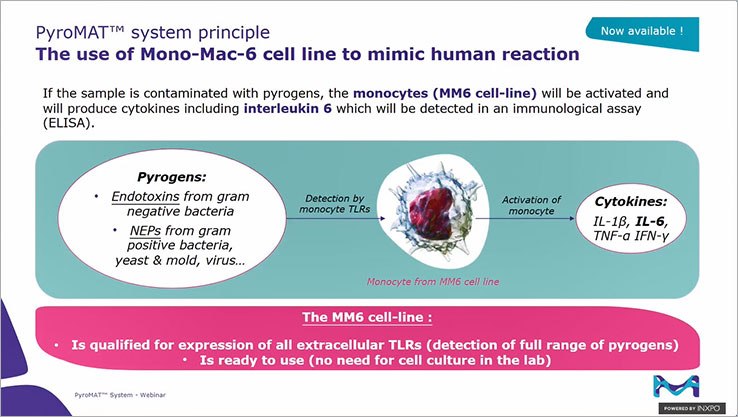
- 符合国际法规和指南: 符合工业和监管机构减少使用动物试验的道德趋势。
- 标准化反应性和高灵敏度(LOD 0.05 EU/mL)。 即用型细胞系的便利性减少了费力的实验室工作,避免了对细胞培养实验室的需求。
- 合格细胞: Mono-Mac-6 细胞除了在 MAT 国际验证中被引用外,还通过了所有表面 Toll-Like Receptors (TLRs) 的表达鉴定,以确保检测广泛的热原。
- 检测多种热原:与兔热原试验(RPT)一样,MAT 可检测内毒素和非内毒素。
- 可检测的产品范围更广: 最常用的方法--RPT、细菌内毒素检测法(BET)和LAL--可检测的产品种类有限。
- 模拟人体免疫反应的体外检测: 用于建立可靠的预测模型,减少动物消耗。
- 符合国际法规和准则:符合行业和监管机构减少使用动物试验的道德趋势。
- 从 8 位捐献者处采集的冷冻保存血液: 尽可能接近人类对热原的免疫反应。
利用我们提供的服务,优化或简化您的热原测试方法,以实现轻松验证和经济高效的测试:
- 应用服务
- 验证服务
- 培训服务
缺乏MAT实施和验证的资源。我们将为您服务!
可行性研究、方法开发、验证及培训服务可支持您的热原测试实施,请与我们的Pyrogen Testing Experts.
相关资源
- Brochure: PyroMAT® and PyroDetect
Used to detect a broad range of pyrogens in parenteral products such as pharmaceuticals, biopharmaceuticals or medical devices, the MAT gives an in vitro alternative to conventional animal testing in accordance with regulatory guidelines.
- Datasheet: Non-endotoxin Pyrogen Positive Controls
Meet regulatory requirements for Monocyte Activation Test (MAT) with our non-endotoxin pyrogen controls designed for the PyroMAT® system
如要继续阅读,请登录或创建帐户。
暂无帐户?