Papain, Cysteine Protease, Properties & Products
E.C. 3.4.22.2

Papaya (Carica papaya)
Physical Properties and Kinetics
Papain is a cysteine protease of the peptidase C1 family. Papain consists of a single polypeptide chain with three disulfide bridges and a sulfhydryl group necessary for activity of the enzyme.
Molecular weight: 23,406 Da (amino acid sequence)16
Optimal pH for activity: 6.0-7.0
Temperature Optimum for Activity: 65 °C22
pI: 8.75 17; 9.55 18 Spectral properties:
λmax: 278 nm 19
Extinction coefficient, E1% = 25 19
Extinction coefficient, EmM = 57.6 (at 280 nm) 20
Unit Definition: One unit will hydrolyze 1.0 µmole of N-α-benzoyl-L-arginine ethyl ester (BAEE) per minute at pH 6.2 at 25 °C.
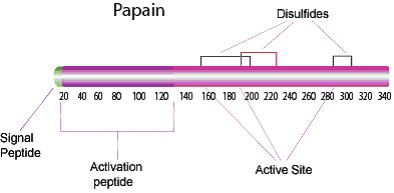
Figure 1.Structure of papain enzyme.
Specificity
Papain will digest most protein substrates more extensively than the pancreatic proteases. Papain exhibits broad specificity, cleaving peptide bonds of basic amino acids, leucine, or glycine. It also hydrolyzes esters and amides. Papain exhibits a preference for an amino acid bearing a large hydrophobic side chain at the P2 position. It does not accept Val at the P1' position.1
Applications
- Papain is commonly used in cell isolation procedures where it has proven more efficient and less destructive than other proteases on certain tissues. For example, papain has been used to isolate viable, morphologically intact, cortical neurons from postnatal rats.2 Our papain preparation (Product No. P4762) has been used for the isolation of smooth muscle cells.3,4 Papain was found to significantly increase the yield of viable smooth muscle cells while not affecting cell sensitivity to stimulants.5
- Limited papain digestion has proven useful for structural studies of enzymes and other proteins.6-8
- Papain is used in red cell serology to modify the red cell surface to enhance or destroy the reactivity of many red cell antigens as an adjunct to grouping, antibody screening, or antibody identification procedures. Papain has also been shown to be useful in platelet serology.9
- Papain has also been used in the enzymatic synthesis of amino acids, peptides, and other molecules.10-13
- Fab and F(ab')2 antibody fragments are used in assay systems where the presence of the Fc region may cause problems. In these cases it is preferable to use only the antigen binding (Fab) portion of an antibody. Papain is used routinely for the preparation of Fab fragments from IgG. IgM may also be digested with papain resulting in high yields of homogeneous Fab preparations.15
- Papain cleaves antibodies into two Fab fragments, which recognize the antigen specifically with their variable region, and one Fc fragment.14 It cleaves above the hinge region containing the disulfide bonds that join the heavy chains, but below the site of the disulfide bond between the light chain and heavy chain. This generates two separate monovalent (containing a single antibody binding site) Fab fragments and an intact Fc fragment. The fragments can be purified by gel filtration, ion exchange, or affinity chromatography. Protocols for antibody digestion and purification of antibody fragments can be found in Antibodies: A Laboratory Manual, E. Harlow and D. Lane, ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y., 1988.
- In tissues such as lymph nodes or spleen, or in peripheral blood preparations, cells with Fc receptors (macrophages, monocytes, B lymphocytes, and natural killer cells) are present which can bind the Fc region of intact antibodies, causing background staining in areas that do not contain the target antigen. Use of Fab fragments ensures that the antibodies are binding to the antigen and not to Fc receptors. These fragments may also be desirable for staining cell preparations in the presence of plasma, because they are not able to bind complement, which could lyse the cells. Fab fragments allow more exact localization of the target antigen, i.e. in staining tissue for electron microscopy.
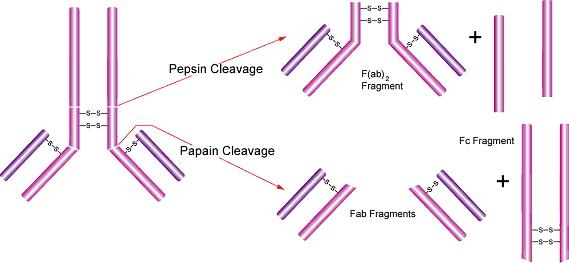
Figure 2.Representation of pepsin and papain cleavage.
Solubility and Solution Stability
Papain is soluble in water at 10 mg/mL. Immediately prior to use, the enzyme is typically diluted in buffer containing ~5 mM L-cysteine. Activation/stabilizing agents include EDTA, cysteine, and dimercaptopropanol.21
Although papain solutions have good temperature stability, the solution stability is pH dependent. Papain solutions are unstable under acidic conditions, i.e., at pH values below 2.8, there is a significant loss in activity. For the active enzyme in solution, the loss in activity is about 1-2% per day, probably as a result of autolysis and/or oxidation.
A common inactive form of papain obtained during isolation is a mixed disulfide formed between the active site sulfhydryl group of the protein and free cysteine.23
Papain solutions are stable to several denaturing agents, i.e., full activity is maintained after recrystallization in 70% methanol and in 8 M urea solutions. However, there is a significant loss in activity when papain is exposed to 10% trichloroacetic acid or to 6 M guanidine hydrochloride.
Related Products
Papain
Inhibitors
Substrates
References
To continue reading please sign in or create an account.
Don't Have An Account?