Vitronectin
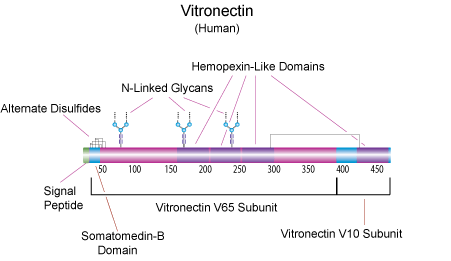
Vitronectin is a glycoprotein present in plasma and tissues. Together with fibronectin, vitronectin is one of the major cell adhesion proteins in plasma. Although these proteins have similar functions and have an Arg-Gly-Asp cell recognition sequence, they are structurally and immunologically distinct. In addition to promoting the adhesion of various cells in culture, vitronectin binds to glycosaminoglycans, is incorporated as an inhibitor to the membrane cytolytic attack complex of the complement system, interacts with thrombin and antithrombin III during coagulation, and may have a physiological role in the coagulation pathway. The N-terminal residues are identical to somatomedin B, which is also present in plasma. This sequence is followed by an RGD sequence, which interacts with a specific cell-surface receptor. Then a sequence of repeat units follows. The central domain of the molecule is enriched with hydrophobic residues. Near the C-terminus is a 12 kDa, arginine rich region responsible for heparin binding activity, expressed after a conformational change. The conformational change occurs in vivo upon binding to the thrombin-antithrombin III complex and in vitro with treatment with urea.
To continue reading please sign in or create an account.
Don't Have An Account?