Glycosaminoglycans and Proteoglycans
Dive into the roles of glycosaminoglycans and proteoglycans including the five types of glycosaminoglycan chains and their structures, biosynthesis, degradation, and functions. Also, browse glycosaminoglycan and proteoglycan products for your research.
Read more about
GAG Structures
Glycosaminoglycans (GAGs) are large linear polysaccharides constructed of repeating disaccharide units with the primary configurations containing an amino sugar (either GlcNAc or GalNAc) and an uronic acid (either glucuronic acid and/or iduronic acid). There are five identified glycosaminoglycan chains (Figure 1):
Hyaluronan is not sulfated, but the other glycosaminoglycan chains contain sulfate substituents at various positions of the chain. The sulfate groups as well as the uronic acids result in the glycosaminoglycan chains having a negative charge. GAG polymers are significantly larger than N-glycans or O-glycans and the chains are linear rather than branched like N-glycans.
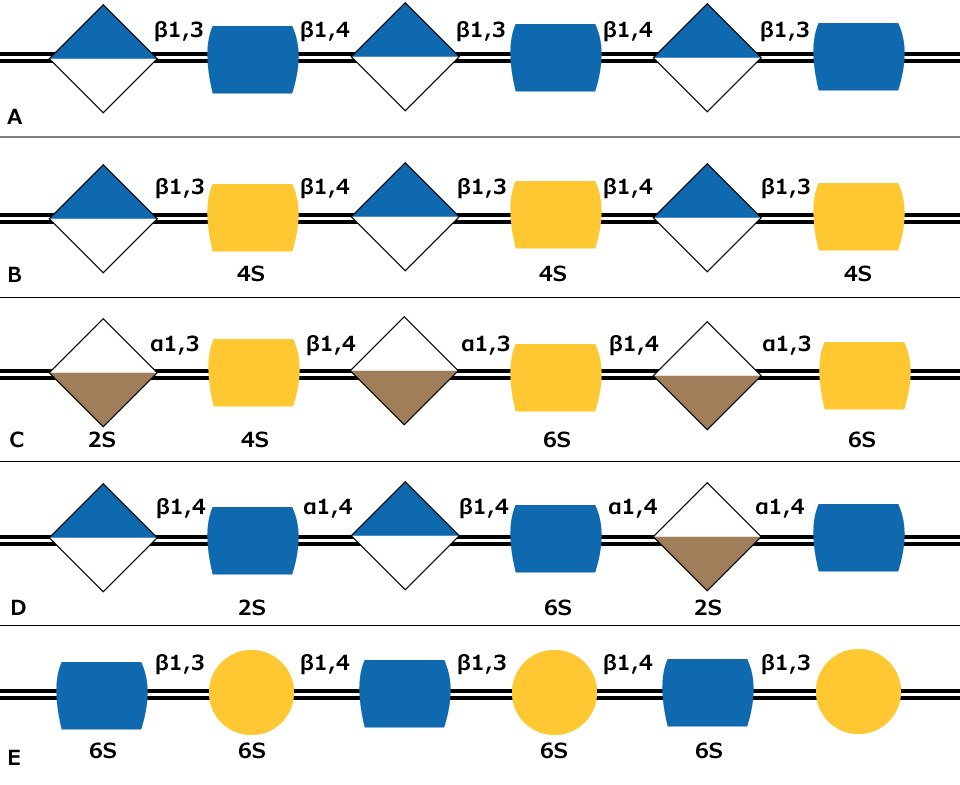
Figure 1. Carbohydrate sequences of the five types of glycosaminoglycan chains using monosaccharide symbols: (A) Hyaluronan, (B) Chondroitin, (C) Dermatan, (D) Heparin and (E) Keratan. Possible sulfation presence and location (2S, 4S, or 6S) is indicated.
Hyaluronan
Hyaluronan (HA; hyaluronic acid) is composed of alternating residues of β-D-(1→3) glucuronic acid (GlcA) and β-D-(1→4)-N-acetylglucosamine (GlcNAc) (Figure 2). Unlike the other glycosaminoglycans, hyaluronan does not attach to proteins to form proteoglycans.
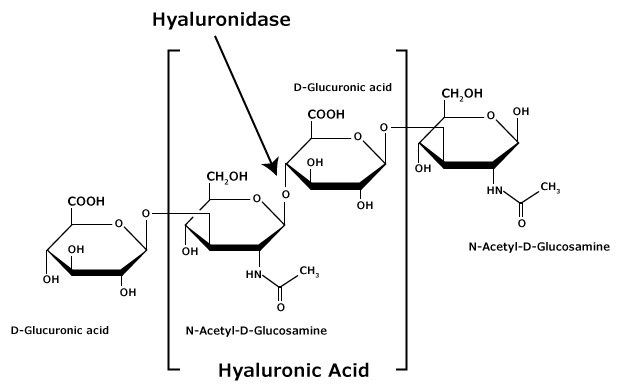
Figure 2.Hyaluronic acid is composed of alternating residues of β-D-(1 3) glucuronic acid and β-D-(1 4)-N-acetylglucosamine.
Chondroitin and Dermatan
Chondroitin sulfate and dermatan sulfate (chondroitin sulfate B) are composed of disaccharide units containing N-acetylgalactosamine (GalNAc) and an uronic acid joined by β(1→4) or β(1→3) linkages, respectively (Figures 3, 4, 5). Chondroitins contain glucuronic acid (GlcA) and are 4‑O-sulfated (chondroitin sulfate A) or 6‑O-sulfated (chondroitin sulfate C). Dermatan sulfate also contains N-acetylgalactosamine (GalNAc), but the uronic acid present in dermatan is L-iduronic acid (IdoA).1
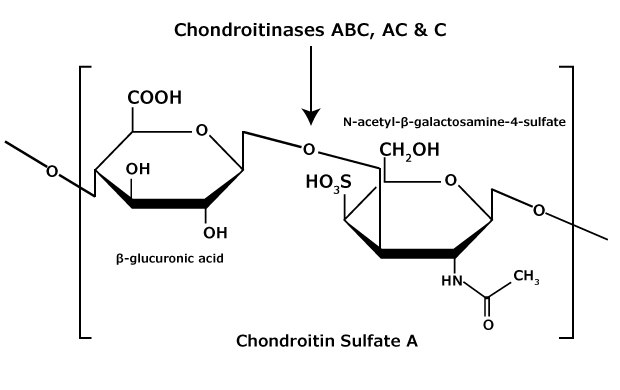
Figure 3.Chondroitin sulfate A consists of an alternating copolymer β-glucuronic acid-(1 3)-N-acetyl-β-galactosamine-4 sulfate.
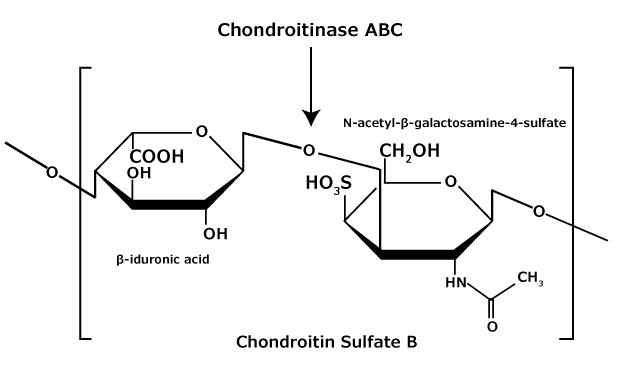
Figure 4.Chondroitin sulfate B (dermatan sulfate) consists of an alternating copolymer β-iduronic acid-(1 3)-N-acetyl-β-galactosamine-4 sulfate.
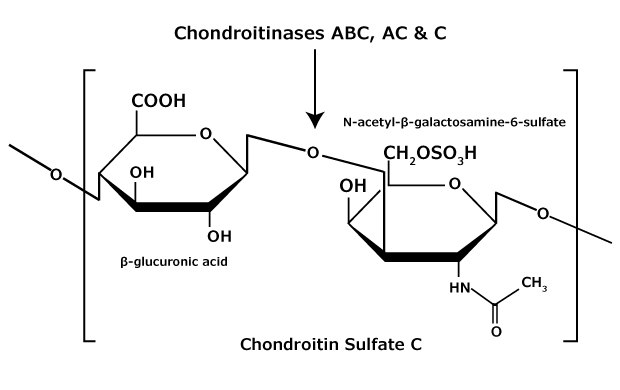
Figure 5.Chondroitin sulfate C consists of an alternating copolymer β-glucuronic acid-(1 3)-N-acetyl-β-galactosamine-6 sulfate.
Heparan and Heparin
Heparan and heparin glycosaminoglycans are complex heterogeneous mixtures of repeating disaccharide units consisting of an uronic acid (D-glucuronic or L-iduronic acid) and D-glucosamine or N-acetyl-Dglucosamine (Figure 6). Various degrees of sulfation occur (at the oxygen and/or nitrogen containing groups) on each monosaccharide unit, ranging from zero to tri-sulfation. Heparan is less sulfated than heparin.
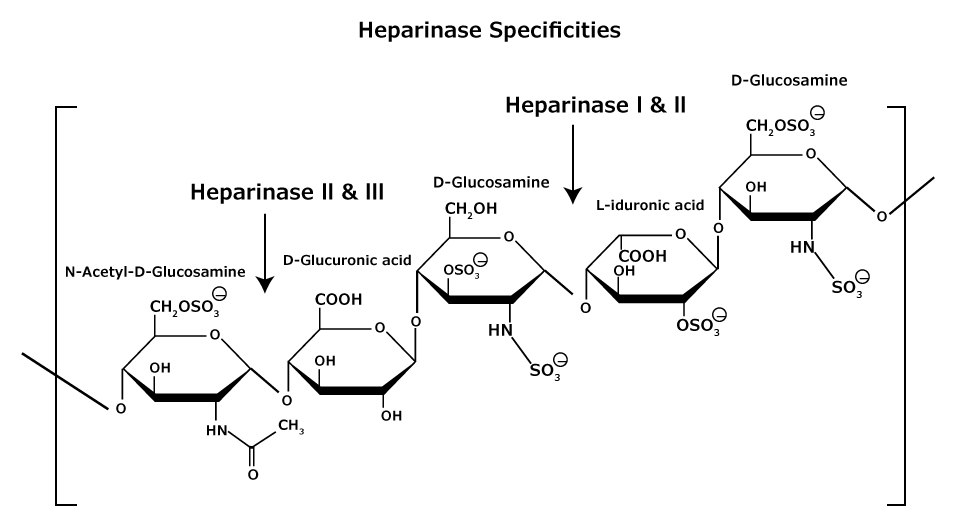
Figure 6.Heparan and heparin glycosaminoglycan consist of heterogeneous mixtures of repeating units of D-glucosamine and L-iduronic acids or D-glucuronic acids, sulfation at each residue may vary.
Keratan
Keratan sulfate differs from the other glycosaminoglycan chains in that it does not contain uronic acid residues. Keratan is made up of Nacetyllactosamine (βGal-β(1→4)-GlcNAc) subunits. It may be attached to the protein backbone through either N-linkage or O-linkage.
Proteoglycans are the specific group of glycoproteins that have at least one glycosaminoglycan chain attached to the protein; categorization is typically by the GAG chain(s) present. Heparan/heparin sulfate and chondroitin sulfate are the most common GAGs contained by proteoglycans. In addition, most proteoglycans also contain N-linked and O-linked glycans.
Biosynthesis and Degradation
The biosynthesis of hyaluronic acid (hyaluronan) is a stereospecific enzymatic copolymerization of N-acetylglucosamine and glucuronic acid. The hyaluronan synthetases involved in this process require UDP-Nacetylglucosamine and UDP-glucuronic acid as sugar donors, and can generate a 1,000,000 Dalton polymer in less than a minute. Hyaluronan is cleaved by hyaluronidases; testicular hyaluronidases degrade hyaluronan to tetrasaccharides, while bacterial hyaluronidases degrade hyaluronan to disaccharides. In lysosomes, hyaluronan is degraded to the monosaccharides N-acetylglucosamine, which is recycled, and glucuronic acid, which is further metabolized via the pentose phosphate pathway.
The biosynthesis of the sulfated glycosaminoglycans involves both polymerization and sulfation steps. Sulfation is performed by sulfotransferases utilizing a 3′-phosphoadenyl-5′-phosphate (PAPS) activated sulfate donor. Keratan chains can be over 20,000 Dalton and contain a series of lactosamine (βGal-β(1→4)-GlcNAc) subunits that are nonsulfated, monosulfated (6‑position of GlcNAc), or disulfated (6‑position of both Gal and GlcNAc). In animals, degradation occurs via the sulfatase-catalyzed removal of the terminal sulfate and the sequential action of exoglycosidases. Bacterial keratanases can degrade keratan sulfate at specific positions.
Both chondroitin sulfate and heparan sulfate classes of sulfated glycosaminoglycans link to serine residues in core proteins through a common tetrasaccharide construct (GlcA-Gal-Gal-Xyl-Ser). This process is initiated by the xylosyltransferase-catalyzed attachment of xylose using UDP-xylose as donor. After the attachment of the tetrasaccharide, the proteoglycan structure diverges, since the next carbohydrate linkage determines the glycosaminoglycan class attached. A β-glycosidic bond to N-acetylgalactosamine results in the attachment of chondroitin sulfate to the peptide, while a α-glycosidic bond to N-acetylglucosamine results in the attachment of heparan sulfate.
Glycosaminoglycan & Proteoglycan Functions
Glycosaminoglycans and proteoglycans have many functions. Hyaluronan is a major component of the extracellular matrix; it binds and retains water molecules and fills the gaps between collagen fibrils. CD44, a human cell surface glycoprotein that participates in multiple cell functions binds to hyaluronan, and hyaluronan-CD44 interactions have been associated with malignant tumor invasion. The interactions of glycosaminoglycans with a variety of ligands are associated with inflammation, growth, coagulation, fibrinolysis, lipolysis and cell-matrix biology.
Proteoglycans are also components of the extracellular matrix, and they interact with a variety of molecules, including cell adhesion molecules and growth factors such as transforming growth factor-β (TGF-β) and basic fibroblast growth factor (bFGF).
Find more technical resources on our Glycobiology hub page.
Glucosaminoglycans
Glucosaminoglycans
GAG Hydrolyases and Lyases
GAG Hydrolyases and Lyases
References
To continue reading please sign in or create an account.
Don't Have An Account?