Gel Electrophoresis with mPAGE® Gels
mPAGE® bis-tris precast gels provide the high resolution you need for publication-ready results, without breaking your budget. Achieving identical protein separation with shorter run times (up to 15 minutes), this affordable solution is compatible with most electrophoresis tanks. Switch to mPAGE® precast gels today to save without sacrificing what you need most.
- Publication-ready resolution at a fraction of the cost
- Compatible with popular electrophoresis tanks
- Up to 80 μL sample per well (with 10-well gels)
- Efficient wet and semi-dry Western blot transfer
- Up to 15-minute shorter run time
- Neutral pH prevents protein modification
- Protein Separation and Membrane Transfer with mPAGE® Precast Gels
- How to Select mPAGE® Precast Gels
- mPAGE® Gels Cross Reference Guide
- Ordering Information: mPAGE® Gels, Buffer, and Accessories
Protein separation and membrane transfer with mPAGE® precast gels
Polyacrylamide gel electrophoresis (PAGE) is a ubiquitous technique used to separate proteins by electrophoretic mobility. While proteins can be separated in their native (or folded) state via native-PAGE, proteins are typically chemically denatured (or unfolded) using sodium dodecyl sulfate (SDS) with a reducing agent like dithiothreitol (DTT) prior to separation. SDS PAGE gels are used to separate proteins based on their molecular weight, comparing samples to standards (ladders) containing proteins of known molecular weight.
Separation
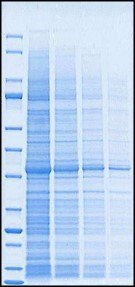
Run time for gel electrophoresis is variable, depending on the gel and running conditions. mPAGE® bis-tris precast gels were shown to have a shorter run time (up to 15 minutes), than similar precast gels available on the market (Figure 1), without losing resolution – helping you save time and money.

Competitor T
50-minute run time
mPAGE® Precast Gel
35-minute run time
Figure 1. Protein separation using MOPS running buffer comparing mPAGE® Bis-Tris Precast Gel (4-12%) to a competitor precast bis-tris gel. Samples: unstained protein marker and serial dilution of E.coli extract.
Transfer to membranes
After separation, proteins are transferred to a membrane, typically for Western blotting (immunoblotting) or another detection technique. In Western blotting protocols, proteins are most commonly electrophoretically transferred either using a wet (tank) or semi-dry transfer method. mPAGE® bis-tris precast gels provide efficient protein transfer for both wet and semi-dry transfer (Figure 2).
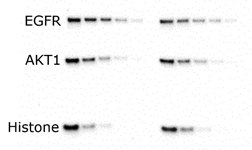
Wet Transfer
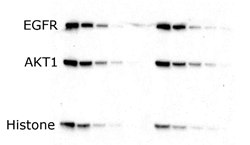
Semi-dry Transfer
Figure 2. Serial dilutions of A431 cell lysate loaded onto an mPAGE® 4-12% gel and transferred to Immobilon®-P membrane via standard methods. Blots probed with antibodies against EGFR (180 kDa), AKT1 (60 kDa) and histone (17 kDa). Antibodies detected with peroxidase-conjugated secondary antibodies and Immobilon® Forte chemiluminescence substrate.
How to select mPAGE® precast gels
Precast gels for SDS-PAGE are selected based upon the size of the proteins being separated. Precast gels are available in different fixed acrylamide percentages, or in gradients where the percentage changes across the gel. Typically, lower percentage gels are used to resolve high molecular weight proteins and high percentage gels are used to resolve low molecular weight proteins. Gradient gels can separate broader protein ranges than fixed percentage gels. The choice of MOPS or MES running buffer can be used for further tuning of the separation. MOPS can provide improved separation at the higher range of the gel, and MES can improve separation at the lower range of the gel. Use the migration charts below for MOPS (Figure 3) and MES buffer (Figure 4) to identify the best mPAGE® gel and buffer for your electrophoresis application.

Figure 3.Migration chart for mPAGE® precast gels using Tris-MOPS SDS running buffer

Figure 4.Migration chart for mPAGE® precast gels using MES SDS running buffer
mPAGE® Gels, Buffers, and Accessories
To continue reading please sign in or create an account.
Don't Have An Account?