Protein Labeling and Purification with Amicon® Pro Centrifugal Filters
Commercially available protein labeling kits provide researchers with the flexibility to customize their own immunoassay detection panels. However, because many protocols require buffer exchange prior to and following antibody labeling, dialysis-based workflows are time-consuming and subject to significant protein loss at multiple points of sample transfer.
For removing unincorporated label in protein labeling applications, centrifugal devices are a fast, convenient, high-recovery alternative to gel filtration.
Small-Scale (25 μg – 200 μg) Antibody Labeling Using the Amicon® Pro Centrifugal Filter
Significantly higher yields can be achieved from antibody labeling by combining the labeling step with the clean-up step in the all-in-one Amicon® Pro system. The Amicon® Pro purification system is an adaptable centrifugal device coupling affinity purification with downstream sample concentration and buffer exchange.
Two attributes of the Amicon® Pro device make it a convenient device for preparing pure, labeled antibody:
- First, the device enables highly efficient buffer exchange via diafiltration with simultaneous sample concentration in a single 15 minute spin.
- Second, the entire workflow can be performed within a single device, reducing the potential for sample loss.
For details on the Amicon® Pro Antibody Labeling application, please visit our dedicated Antibody Labeling page, as well as our publication (Read our 2015 paper in Journal of Immunological Methods)
Read below for a brief summary of the protocol for using the Amicon® Pro device for small–scale antibody labeling.
NOTE: All steps, with the exception of binding reactions, are spin-based. A swinging bucket rotor is required for all steps with the exception of the reverse spin.
Optional: Pre-wet device using 0.5 mL TBS-T. Spin at 1000 x g for 1 min.
- Load antibody solution into the Amicon® Pro device as follows:
For dilute antibodies (< 1 mg/mL), attach an Amicon® Ultra 0.5 mL filter (10k MWCO) to the base of the Amicon® Pro device. Load antibody (up to 1 mL) into the exchange device. Spin 4000 x g for 15 min. - For concentrated antibodies (≥ 1 mg/mL), load sample (up to 100 µL) into an Amicon® Ultra 0.5 mL filter (10k MWCO). Attach filter to the base of the Amicon® Pro device.
- Prepare a 1.5 mL reaction cocktail containing dye in appropriate reaction buffer. Add cocktail to the exchange reservoir.
- Spin 4000 x g for 15 min.
- Optional: Incubate sample for an additional 30 minutes at room temperature, if necessary for optimal labeling efficiency. Read our publication for more details.
- Add 1.5 mL phosphate-buffered saline (PBS) ± Na Azide to the exchange reservoir.
- Spin 4000 x g for 15 min to exchange buffer and concentrate.
- Recover labeled antibody from the Amicon® Ultra 0.5 filter by reverse spin.
Sample Results of Antibody Labeling Protocol
To compare the functional performance of antibodies labeled using traditional centrifugal diafiltration and using the Amicon® Pro method, their detection ability was compared with that of a species/anti-species GAPDH detection pair. Using similar amounts of primary antibody for each blot, both biotin/SA pairs demonstrated the same, if not slightly better sensitivity, than the species/anti-species detection pair (Figure 1).
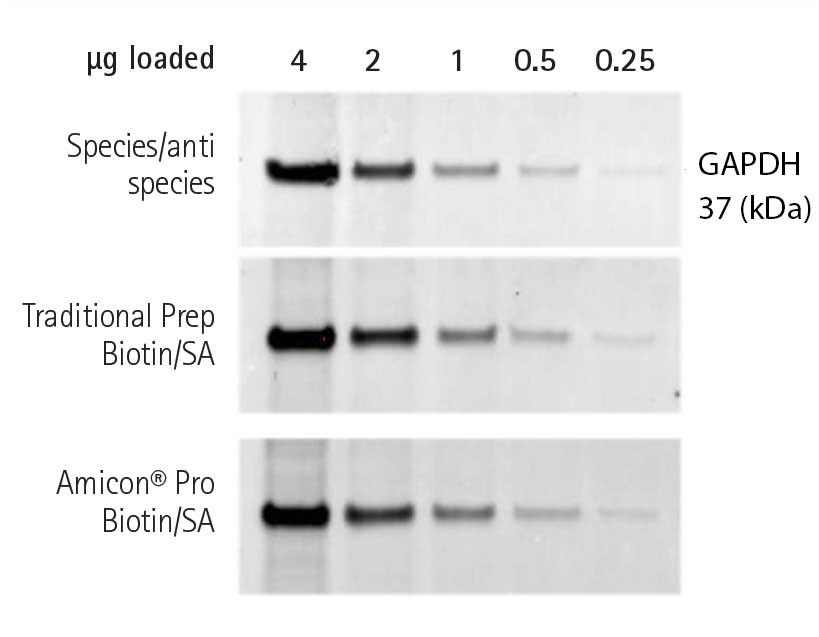
Figure 1.
Anti-GAPDH, biotinylated using the Amicon® Pro system, was used to successfully detect GAPDH in Western blots. Aliquots of EGF-stimulated A431 cell lysate (0.25-4 µg) were resolved by SDS-PAGE, transferred onto Immobilon®-P membrane, and probed for β tubulin in the SNAP i.d.® 2.0 system using the various indirect detection pairs. In each case, the same quantity of primary (GAPDH-specific) antibody was used.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?