Nitrosamine Impurities Testing by LC-MS Methods from the United States Pharmacopeia General Chapter <1469>
Introduction
Nitrosamines are unwanted side products present in many substances and are suspected to have toxic and carcinogenic properties. In pharmaceutical raw materials and finished drug products, nitrosamines may also be formed as side-products from synthesis, during storage, or from packaging, etc. A demand for nitrosamine analysis has rapidly increased worldwide. The list of nitrosamine impurities manufactured from drug substances using specific synthetic routes has grown after extensive synthetic route assessments.
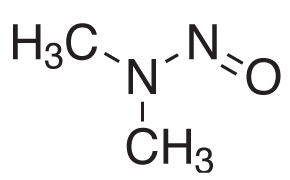
Figure 1.Chemical Structure of N-Nitrosodimethylamine (NDMA).
The United States Pharmacopeia (USP) published in December 2021 new procedures in response to the unexpected detection of nitrosamines, such as N-nitrosodimethylamine (NDMA) impurity, Figure 1, in certain active pharmaceutical ingredients (API) and corresponding final formulations.1 The new USP chapter <1469> provides recommendations regarding the creation of controls of nitrosamine impurities limits to ensure their elimination or reduction, and analytical method performance characteristics for nitrosamine testing procedures using both GC-MS (procedures 2 and 4) and LC-MS (procedures 1 and 3).
This paper focuses on the LC-MS based test procedures (procedure 1 and 3) for quantitative analysis of known nitrosamine impurities in drugs and pharmaceutical raw materials using liquid chromatography and mass spectrometric detection. Even though both methods were evaluated, final run conditions and data from the procedure 3 will be presented. System suitability criteria for the procedure 1 could not be met with the instrumentation available, but will be described.
Procedure 1 designates the use of a high-resolution mass spectrometer (HRMS) and can be used for the quantitation of NDMA, NDEA (nitrosodiethylamine), NDBA (nitrosodibutylamine), NDIPA (N-nitrosodiisopropylamine), NEIPA (N-nitrosoethylisopropylamine), NMBA (N-nitrosomethylaminobutyric Acid), and NMPA (N-nitrosomethylphenylamine) in selected sartans (valsartan, irbesartan, and losartan potassium). Procedure 3 uses MS/MS and can be used for the quantitation of NDMA, NDEA, NDIPA, NEIPA, NMBA, and NDBA in selected sartans (valsartan, losartan potassium, olmesartan medoxomil, candesartan cilexetil, and telmisartan).
Experimental Conditions
Sample and Standard Preparation
The used standard and sample solutions were prepared as follows:
- Internal Standard Solution: 10 μg/mL each of NDMA-d6 and NMBA-d3, as well as 1 μg/mL each of NDEA-d10 and NDBA-d18 were prepared in water
- Nitrosamine standards stock solution mixture: A mixture containing 200 ng/mL each of NDMA, NEIPA, NDIPA, NDBA and NMBA was prepared by mixing appropriate volumes of the respective USP Reference Standards and diluted with water.
- NDEA standard stock solution: A solution of 132 ng/mL of NDEA was prepared by diluting USP N-Nitrosodiethylamine RS with water.
- Standard solutions: Depending on the targeted nitrosamine concentration in the sample, a set of 5 consecutive linearity solutions were prepared as described in Table 1 from the nitrosamine standards stock solution mixture and NDEA standard stock solution by mixing specified volumes of each solution as indicated.
- The samples were prepared as follows:
- 80 mg of the drug substance transferred into a 2 mL lidded centrifuge tube.
- Addition of 1188 μL diluent (1% formic acid in water) and 12 μL of the Internal standard solution.
- Vortexing at 2500 rpm for 20 min (except for losartan potassium, which should be vortexed NMT 5 min).
- Centrifuging at about 10,000 rpm for 10 min
- Filtering into a vial using a PTFE filter with 0.45-µm pore size.
Valsartan Samples
The valsartan sample solution was prepared following the sample preparation protocol. “Valsartan” in Table 8 means drug product (tablet/tablet powder).
Losartan Samples
The losartan sample solution was prepared following the sample preparation protocol. “Losartan” in Table 10 corresponds to losartan tablets or ground losartan tablets
LC-MS (Procedure 3)
Separations were conducted on an Agilent 1290 Infinity II HPLC system (Agilent, Waldbronn, Germany) equipped with a 6495C triple quadrupole MS detector having an APCI Source. Chromatographic separations were performed in gradient mode on an Ascentis® Express C18 (USP L1 Packing) 150×3.0 mm I.D., 2.7 µm column (see Table 2-4).
Data Handling
Data acquisition and processing were performed using Masshunter software version 10.0.
Results and Discussion
Evaluation of Procedure 1
The USP procedure 1 method describes the use of liquid chromatography and high-resolution mass spectrometric detection (LC-HRMS), but the given experimental conditions1 appear not generic enough to allow implementation and validation on any given HRMS platform. Our laboratory experienced sensitivity issues with an ultra-modern HRMS detector (Agilent 6546 Q-TOF), and to verify the results different HPLC columns, solvents, and reagents were tested. Chromatographic system suitability criteria could be met, but it was not possible to meet system suitability for overall identification and sensitivity.
Comparable signal intensities were attained at 1 µg/mL, 100 ng/mL, and 50 ng/mL for NDEA, NEIPA, NDIPA, NDBA, and NMPA (ESI positive mode) with a Supelco® L43 column (Ascentis® Express F5), see Figure 2 and two other manufacturers L43 columns (not shown). NDMA impurity was not detected with ANY column at all concentration levels, and NMBA was not detected with any column in ESI(-) mode. The comparison of different L43 columns (pentafluorophenyl groups chemically bonded to silica particles by a propyl spacer) showed similar overall behaviour with procedure 1 using the LC-HRMS instrument, but it was not possible to meet the system suitability for identification and sensitivity. More recently, there has been further clarification posted on the USP Pharmacopeial Forum (USP-PF) regarding procedure 1. It mentions that analyses were performed and validated with an Orbitrap Fusion Lumos Tribrid brand of mass spectrometer. Since this type of instrumentation was not available in our laboratory, further validation of procedure 1 was not pursued.
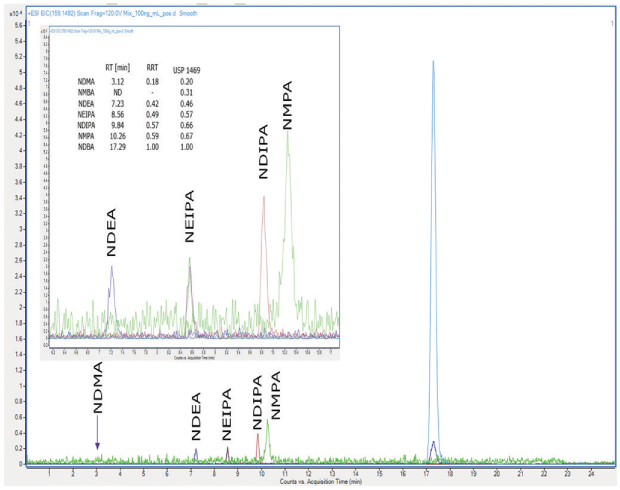
Figure 2.Chromatogram of a 100 ng/mL nitrosamine mixture analysed with a Supelco® L43 column (Ascentis® Express F5), ESI(+) mode.
Evaluation of Procedure 3
This method describes the use of liquid chromatography and tandem-mass spectrometric detection (LC-MS/MS), for the quantitation of NDMA, NDEA, NDIPA, NEIPA, NMBA, and NDBA in selected sartans (valsartan, losartan potassium, olmesartan medoxomil, candesartan cilexetil, and telmisartan). The procedure listed two system suitability criteria: 1. Correlation coefficient: NLT 0.99 and 2. y-Intercept: Not more than (NMT) 25% of the response of the medium concentration solution used in standard curve generation. Going forward, analytical data will be presented from the work establishing a validated analytical procedure 3 using a 150 x 3.0 mm Ascentis® Express C18 column (USP L1 packing) with 2.7 μm particles. An example of a nitrosamine impurity standard on this column is shown in Figure 3.
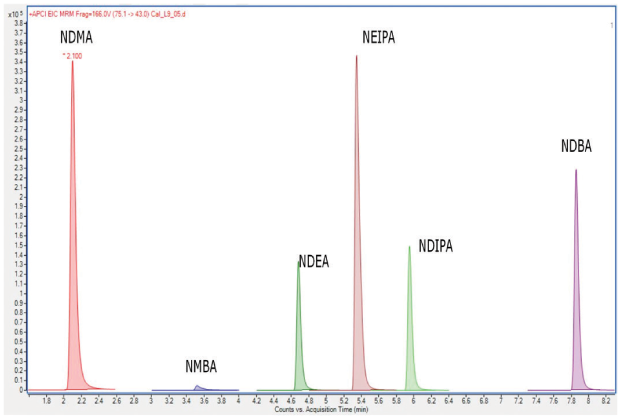
Figure 3.MRM Chromatogram (no scaling) of a 90 ng/mL nitrosamine standard solution using an Ascentis® Express C18 column for procedure 3.
The method linearity was determined over nine calibration levels after optimizing the instrumental set-up. Triplicate injections were made of each linearity solution. The USP Chapter <1469> has defined two system suitability requirements for procedure 3. The correlation coefficient should not be less than (NLT) 0.99 and the y-Intercept for each calibration graph should not be more than (NMT) 25% of the response of the medium concentration solution used in standard curve generation. As shown in Table 5 both of these requirements were met.
The method precision (Table 6) and accuracy (Table 7) were determined using data from ten injections of calibration levels 1, 5 and 9 (L1, L5 and L9). Accuracies of the level 1, 5 and 9 solutions were calculated using the 9-point calibration curves described in Table 1.
The methods limit of detection (LOD) and limit of quantification (LOQ) were determined by spiking 3.3 ng/mL (2.2 ng/mL for NDEA) into a valsartan/ losartan sample solution and using the signal-to-noise (S/N) ratio the for calculation. The limit of detection is defined as a signal-to-noise S/N ratio of 3, whilst the limit of quantification is defined as a S/N ratio of 10. The S/N ratio was calculated by the instrument software, and where the S/N ratio for each peak is established automatically using the peak height and a defined region of noise. Resulting limits for the measured samples are shown in Table 8-11.
The method specificity was determined by monitoring the analytes retention time, and their relative retention to the retention of NDBA for a series of injections of nitrosamine standard solutions (n=40).
The analyte recovery was determined in one valsartan batch and in one losartan potassium batch (Table 13). The drug substance batches were spiked with all analytes over three concentration levels as triplicates during the sample preparation procedure. The prepared sample solutions were measured and evaluated against an external calibration curve to calculate the individual analyte concentration. The ratio of internal standard signal versus analyte signal was determined in the sample solution and in the solutions of the (external) calibration row, i.e., signal NDMA-D6 / signal NDMA. Then the signal ratios were used to calculate the concentration in the sample solution against the calibration solutions.
During the determination of the analyte recovery, a systematic issue with the determination of the spike recovery for NDBA was observed (data not shown), as the found concentrations of this analyte were always too high (recoveries > 130% and thus excluded).
The analysis of possible reasons for this issue showed that coelution with one or several unknown substances occurs during the elution of NDBA.
Conclusion
This paper shows intriguing findings from work with USP Chapter <1469>, Procedure 1, and a successful implementation of Procedure 3 meeting all system suitability requirements.
For a determination of N-nitrosamines in valsartan by GC-MS/MS see the article Determination of N-Nitrosamines in Valsartan
More information on pharma QC topics can be found at SigmaAldrich.com/PharmaQC
Product List (USP Chapter <1469>, Procedure 1)
Product List (USP Chapter <1469>, Procedure 3)
References
To continue reading please sign in or create an account.
Don't Have An Account?