Lentivirus Platform: Optimizing Performance for De-Risked Production
Read more about
- Maximizing Lentiviral Vector Titers Through Upstream Optimization
- Enriching Lentiviral Vector Recovery Through Downstream Optimization
ADDRESSING THE NEEDS OF CELL AND GENE THERAPY INNOVATORS
As part of our comprehensive viral vector development and manufacturing services, we offer a de-risked, reproducible and scalable platform that provides an efficient approach to GMP manufacturing for lentiviral vectors .
The VirusExpress® lentivirus platform delivers a leading solution for suspension-based manufacturing. The platform workflow was intentionally designed to ensure optimized and de-risked lentivirus production and to better address the needs of cell and gene therapy innovators. Our comprehensive platform offers economical value and high quality and performance through:
- Industry-leading titer
- Robust recovery
- Streamlined, scalable workflow
- Comprehensive analytics
- Reduced development timelines to less than 12 months

Upstream Process
- Cell growth
- Transfection
- Virus production

Downstream Process
- Harvest & clarification
- Chromatography
- UF/DF concentration
- Sterile filtration
- Formulation

Analytics
- Lentivirus product
- Host cell impurities
- Compendial testing
- Potency
De-Risking Lentiviral Vector Manufacturing with Optimized Upstream and Downstream Processes
Through its optimized and defined processes, the streamlined platform accelerates development timelines (as seen in Figure 1) and delivers industry-leading titers and robust recovery yields. The comprehensive analytics provide performance and product quality data to ensure that manufacturing is robust.
The VirusExpress® platform utilizes an optimized transfection-based solution for upstream production with VirusExpress® 293T Lentiviral Production Cells, a well-characterized, GMP-banked HEK293T cell line and EX-CELL® CD HEK293 Viral Vector Medium, a chemically-defined cell culture medium.
The platform`s upstream and downstream workflows were optimized using design of experiments (DoE) and supplementary studies. The results of these studies include data on scalability and reproducibility outcomes.
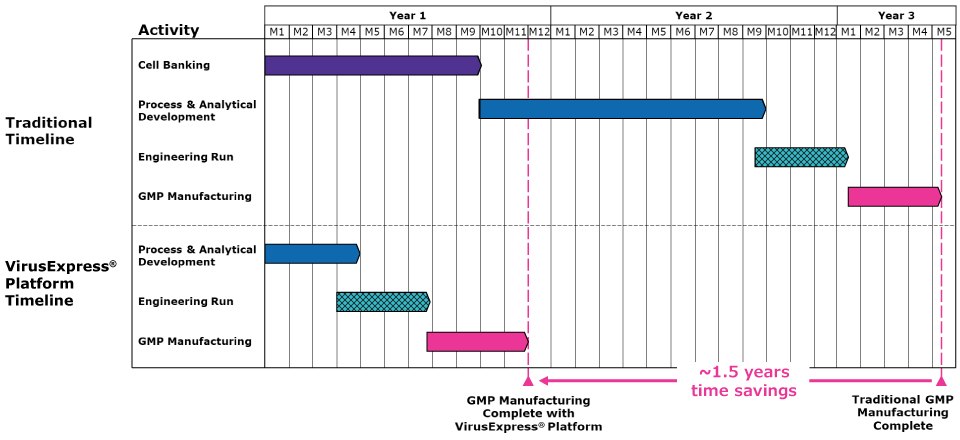
Figure 1.VirusExpress® platform for lentiviral vectors delivers significant time and cost savings compared to a typical custom process. The time required for production of LV vector can be reduced by more than one year.
Maximizing Lentiviral Vector Titers through Upstream Optimization
To identify the impact of transfection parameters on infectious titer compared to control conditions, two DoE studies were performed. In the first study, thirty-three different test conditions, as well as controls, were evaluated simultaneously. High, medium, and low values for five parameters were assessed. Using this approach, infectious titers as high as 6.3E+08 infections units (TU)/mL were achieved (Figure 2A).
To ensure optimal LV production, an experimental approach was used to identify the ideal levels of each plasmid added during transfection, given that multiple plasmids are utilized. A mixture design experiment was conducted, in which the levels of each plasmid were varied to ensure the defined amount of input DNA was consistent. In total, 30 test samples were evaluated during the experiment. (Figure 2B).
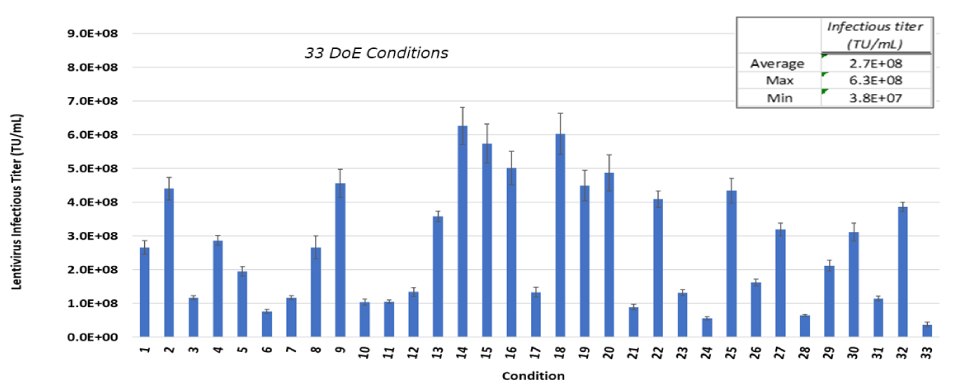
Figure 2A.A DoE study with 33 different study conditions to optimitze for infectious titer. Each bar represents an average of two dilutions of lentivirus plated in triplicate and error bars are standard deviations.
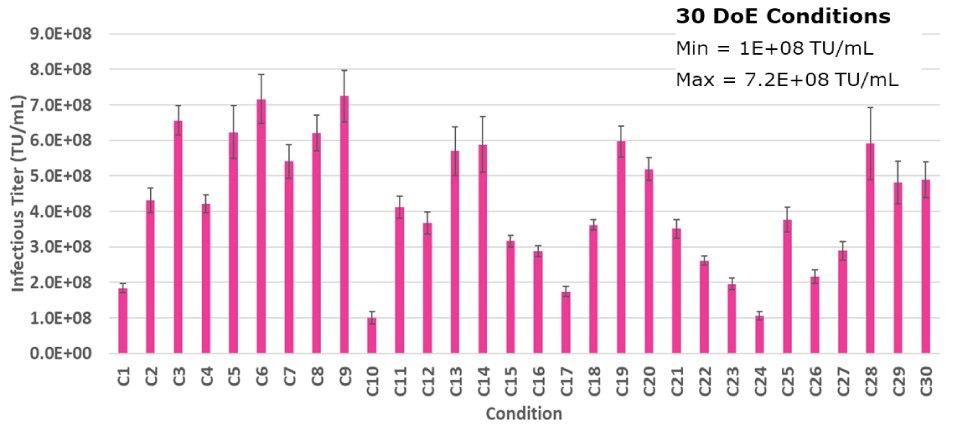
Figure 2B.A DoE study displaying the 30 different outcomes for optimal plasmid molar ratios. Each bar represents an average of two dilutions of lentivirus plated in triplicate and error bars are standard deviations.
Using the identified optimal transfection conditions, testing was performed at larger scales to confirm the robustness of the upstream workflow. The scale-up of LV vector production, based on DoE studies, is also included in the analysis.
Enriching Lentiviral Vector Recovery Through Downstream Optimization
To improve the downstream workflow and maximize recovery of LV vectors, the downstream optimization studies focused on anion exchange (AEX) chromatography, tangential flow filtration (TFF), final formulation, and sterile filtration unit operations, which had been identified as having the lowest step recoveries.
Step Elution Screening
To optimize NaCl concentration in the elution step and determine which condition enabled the highest infectious particle recovery, a step elution from 0.5 M NaCl to 2M NaCl was evaluated (Figure 3A). A total of 79% of the infectious titer was recovered from the combined step elution fractions; no measurable p24 was detected above the 1.5M salt concentration. Total HCP removal was >98% and there was no detectable DNA in the elution fractions (data not shown). Figure 3B shows the conductivity and UV absorbance for each salt concentration in the step elution and that no UV absorbance was detected at 2M NaCl elution. Based on these results, a single-step elution between 1 - 1.5M NaCl was selected, and further experiments refined the ideal elution conductivity between those concentrations. Optimization of the AEX step improved recovery to 88% with consistent performance while retaining the desired level of HCP removal.
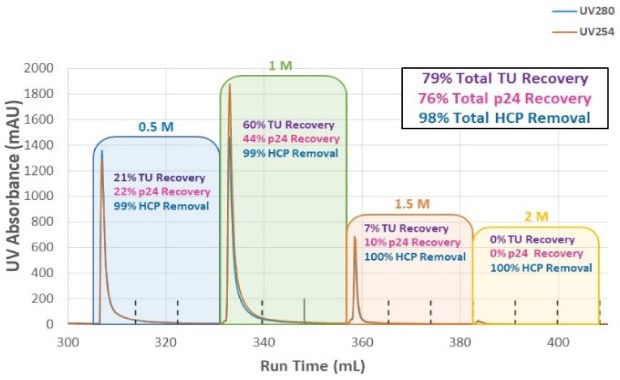
Figure 3A.Step elution recovery and HCP removal in each salt concentration and overall recovery for varying salt concentrations.
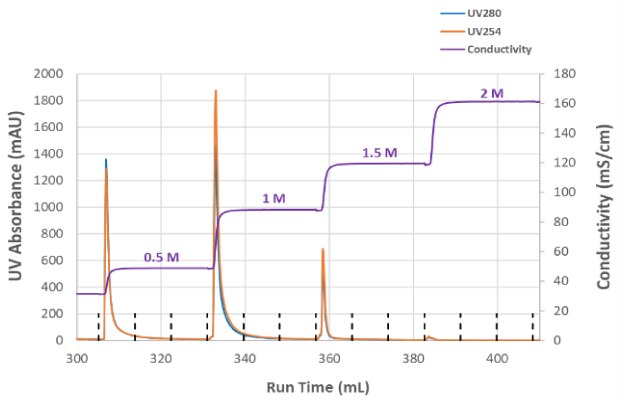
Figure 3B.Step elution conductivity conditions and UV absorbance for varying salt concentrations.
Comparison of Membrane and Device Format on Recovery
TFF was optimized both for concentration and diafiltration. Several device types and formats, including hollowfiber and cassettes, were evaluated, as well as different membrane molecular weight cutoffs (MWCO) from 100 kDa to 750 kDa. The process was optimized for shear rate, duration, and critical flux. Another objective was to monitor the permeate for any breakthrough. No breakthrough was detected in the permeate (less than 1% or below assay limits of 1E5 viral particles/mL). A high recovery was achieved across MWCO and device type from 100 - 500 kDa (Figure 4). This result highlights the flexibility within the platform operating range and the high level of performance of TFF device type.
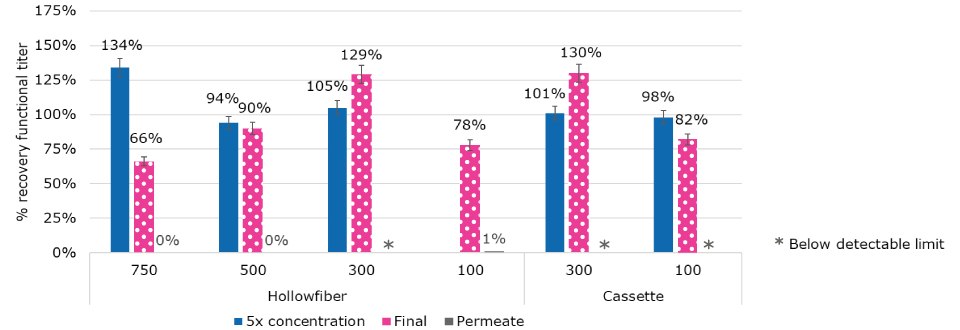
Figure 4.Recovery from TFF operations was optimized across device format and membrane MWCO.
Developing a Final Formulation Buffer
For the formulation buffer optimization, a range of solutions were tested to understand the effect of pH, salt concentrations and stabilizers, hold times, and temperatures, which may occur during the final processing, storage of the LV vector. Figure 5 shows a range of varying buffer formulations and highlights two that provided a 2-fold improvement in recovery at different temperature and time periods.
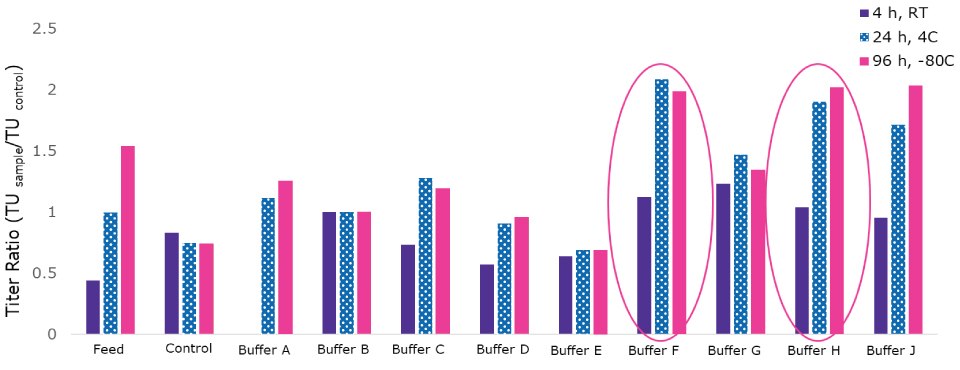
Figure 5.Final formulation buffer development.
The downstream workflow improvements were processed at larger scales to confirm consitent recovery and yield. The downstream production outcomes of LV vectors are included in the results, which can be found in our whitepaper: Optimizing and Scaling Lentiviral Vector Production
Analytical Assays to Support Lentiviral Vector Production Process
Accurate measurement of virus particles and impurities are essential during harvest and purification to monitor and assess viral vector critical quality attributes (CQAs), in addition to compendial and final product sterility and stability testing. As part of the lentivirus platform, a comprehensive toolbox of analytical methods was defined to characterize the product and assess productivity, yield, and purity.
To continue reading please sign in or create an account.
Don't Have An Account?