Materials for Advanced Thermoelectrics
Thermoelectric materials comprise a wide range of solid compounds distinguished by their ability to convert thermal and electrical energy. This property gives rise to two distinct technological applications: the development of temperature gradients for heating and cooling devices and the generation of electrical energy from waste heat.
The conversion efficiency of a thermoelectric material is related to the dimensionless figure of merit, ZT (Equation 1):
ZT = S2σT/κ
S = Seebeck coefficient
σ = electrical conductivity
κ = thermal conductivity
T = absolute temperature
The Seebeck coefficient, or thermopower, is a measurement of the amount of voltage generated per unit of temperature difference and is typically given in μV/K. Accordingly, obtaining a high figure of merit thermoelectric requires maintaining high electrical conductivity and large thermopower while simultaneously limiting thermal conductivity.
As shown in Figure 1, a typical thermoelectric module contains both n-type and p-type thermoelectric materials connected in series. N-type materials possess electron charge carriers and have negative Seebeck coefficients; conversely, p-type materials possess positive Seebeck efficients and have hole charge carriers. Applying a temperature gradient across the module causes the carriers to diffuse towards the cold side, generating a thermoelectric voltage.
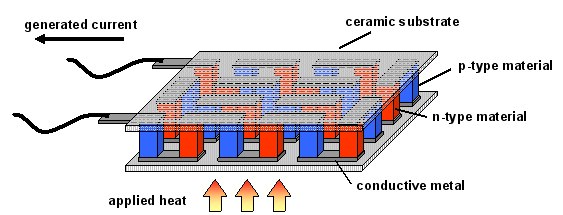
Figure 1.Schematic diagram of a typical thermoelectric module. Small legs of n-type (red) and p-type (blue) materials are connected in series and then sandwiched between ceramic substrates. In the case of electrical generation, heat is applied to one side of the module, causing the charge carriers to diffuse across the module and generating an electrical current.
ZT is dependent on the module operating temperature and has remained approximately 1 for the past several decades for archetype materials at all temperature ranges. These materials include antimony and bismuth tellurides for room temperature applications (300 K), lead telluride (Product No. 254266) and skutterudite antimonides at moderate temperatures (650 K), and silicon-germanium alloys at high temperatures (1000 K).
One of the primary challenges in developing advanced thermoelectric materials is decoupling S, σ, and κ, which are typically strongly interdependent. One approach to this is reducing the lattice contribution to the thermal conductivity through nanostructuring. Heat-carrying phonons can be scattered by introducing a large number of grain boundaries at the nanoscale. This method has yielded a significant increase in ZT for Bi-Sb-Te alloys.3 In this study, nanograined powder was produced by mechanical attrition of bulk ingots. The resulting powder was consolidated through high temperature pressing under vacuum, producing a bulk sample with a ZT of approximately 1.5. Coherent nanoscale inclusion phases in the PbTe-AgSbTe pseudobinary system have also yielded low thermal conductivities and high values of ZT (>1). This was achieved by producing a material with nanoscale precipitates within a bulk matrix.4 Finally, the synthesis of thermoelectric materials with tailored electronic structures has shown promising results, yielding a ZT of approximately 1.5 for thallium-substituted PbTe.5
Bulk scale synthesis of thermoelectric materials is typically approached through standard metallurgical techniques, such as powder metallurgy and synthesis from a high temperature melt. High purity elemental precursors (see list below) are essential in controlling physical properties, as impurities can negatively affect conductivity and the concentration of charge carriers. Furthermore, the use of high purity starting materials allows for consistent and precise control over the composition of the final material, which is essential for studies involving intentional doping/substitution and the tailoring of electronic band structures.
Other approaches to thermoelectric materials include solution-phase routes, including solvothermal synthesis and polyol reduction routes. These processes can vary drastically between choice of solvent, surfactant, and reducing conditions, but all focus on the precipitation of discrete powders from cationic solutions of metal organic and metal halide salts, such as bismuth nitrate (Product No. 254150) and lead acetate (Product No. 316512).6-7 Chemical routes toward thermoelectric materials can directly access nanoscale materials, again focusing on decoupling electrical and thermal conductivities and reducing the thermal component through phonon scattering.
The continued demand for reliable sources of energy can be addressed by the discovery of new sustainable energy sources as well as increasing the efficiency of power generation technology. Thermoelectric materials are one approach towards alternative energy and could drastically increase the efficiency of an engine cycle by converting waste heat into electrical energy. This approach to energy scavenging might also prove useful for reclaiming lost energy from automotive exhaust and electronic equipment.
Related Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?