MULTI-seq Sample Multiplexing for Single Cell Analysis and Sequencing
Single-cell RNA-seq (scRNA-Seq) is a powerful but relatively low-throughput tool to analyze gene expression at the single-cell level. MULTI-seq was developed to multiplex sample types using lipid-modified oligonucleotides (LMOs) complexed with unique DNA sample barcodes, allowing for multiple samples to be pooled together in the same single-cell analysis workflow. Sample multiplexing reduces associated costs and provides the additional power of identifying artifacts such as cell doublets in single-cell sequencing and single-nucleus sequencing (snRNA-Seq) applications.
MULTI-seq LMOs anchor DNA sample barcodes on the cell membrane or nuclear envelope of any live cell while preserving cell viability and endogenous gene expression patterns for single-cell multiplexing. MULTI-seq LMO-labeled cells or nuclei are directly used for single-cell workflows, such as Chromium X (10x Genomics) 1. MULTI-seq sample barcodes are separated from endogenous cDNA libraries by size during preparation and can be sequenced independently or as fraction of an endogenous cDNA library. MULTI-seq is suitable for multiplexing applications of primary human mammary epithelial cells, cryopreserved tumors, metastatic sites isolated from a patient-derived xenograft mouse model, peripheral blood mononuclear cells (PBMCs), ZipSeq, mouse retina, and mouse lung 2-5.
The MULTI-seq System and Next-Generation Sequencing (NGS)
The MULTI-seq system consists of lipid-modified anchor oligos (3'-lignoceric acid amide), co-anchor oligos (5'-palmitic acid amide), and DNA sample barcodes (Figure 1A-B). The anchor oligo and a sample-specific DNA barcode are pre-mixed to allow hybridization and then added to washed cells, where the hydrophobic anchor portion localizes the DNA sample barcode to plasma membranes. Subsequent hybridization of co-anchor prolongs the membrane retention of the oligo complex for at least 2 hours in tested cells (Figure 1C-E and Figure 2A). The design of the DNA sample barcode is flexible and can be optimized depending on the single-cell analysis platform and cDNA library kit.
Here we describe a process for capturing DNA sample barcodes along with mRNA molecules from single cells. The DNA sample barcodes include a 3' poly-A (30 bases), a sample barcode (8 bases), and a 5' PCR handle that is necessary for library preparation and anchor hybridization (Figure 1B). The 3' poly-A domain mimics endogenous transcripts, thereby being bound by mRNA capture beads in droplets. The bead-captured DNA sample barcodes are linked with single-cell barcodes and UMIs in droplets, similar to endogenous mRNAs, and carried through the reverse transcription and cDNA amplification steps in common single-cell workflow. MULTI-seq sample barcodes (DNA sample barcode + single-cell barcode + UMI) and endogenous cDNAs are separated by size selection before NGS library construction. The MULTI-seq sample barcode libraries are then constructed by PCR to add the NGS sequence adaptors, such as P5 and P7 (Figure 1F).
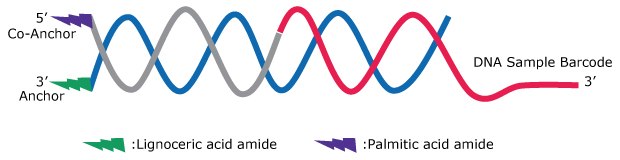
Figure 1A.Scheme for MULTI-seq Lipid Modified Oligos reagent. Membrane embedded Anchor/Co-anchor pair annealed to the DNA sample barcode.

Figure 1B.DNA sequences of Anchor, Co-Anchor and DNA sample barcodes.

Figure 1C.Labeling of MULTI-seq LMO with DNA sample barcode. Step 1: Mix anchor and DNA sample barcode for hybridization.

Figure 1D.Labeling of MULTI-seq LMO with DNA sample barcode. Step 2: Treat anchor/barcode mixture into cells or nucleases. Incubate for 5 minutes on ice.

Figure 1E.Labeling of MULTI-seq LMO with DNA sample barcode. Step 3: Treat co-anchor/barcode mixture into cells or nucleuses. Incubate for 5 minutes on ice. Wash with 1% BSA to absorb excess anchors and co-anchors.
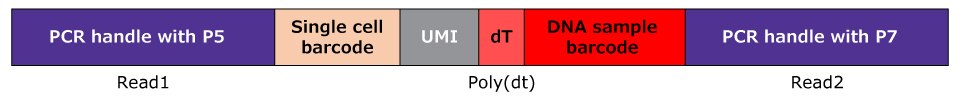
Figure 1F.Completed structure of the MULTI-seq sample barcode library following adapter addition. The DNA sample barcode is captured with poly(dT), mRNA, and the single cell barcode and UMI sequences are linked to DNA sample barcode in a single cell workflow. DNA sample barcode fractions are separated from the endogenous transcript cDNA by SPRI bead selection, while NGS adaptors such as P5 and P7 are added by PCR before sequencing. The size of library DNA will be detected at 180~200 bp position.
The MULTI-seq sample barcode library can be sequenced as a fraction of an endogenous cDNA library or independently. The published protocol is based on the Chromium Single Cell 3´ reagent kit (V2 and V3), and the universal I5 primer is used for the construction of the MULTI-seq sample barcode library 1. Optimization is required for the PCR primer(s) depending on the oligo sequence of mRNA capture beads or library kits when different single-cell technology or cDNA library kits are used. For example, when the Nadia scRNA-seq kit (Nadia instrument, Dolomite Bio) is used, a New-P5-SMART PCR hybrid oligo should be used for the construction of a MULTI-seq sample barcode library instead of the universal I5 primer. Of note, MULTI-seq LMOs provide the technology to locate the DNA sample barcode for sample multiplexing, which is flexible and can be customized depending on applications.
2. Dilute co-anchor to 2 µM concentration in PBS (-).
3. Prepare 4 mL per sample of 1% BSA in PBS (-) and place on ice.
Preparation of cells:
- Prepare single cell suspension in PBS (-). Final concentration is ~500,000 cells in 180 µL. If PBS (-) is not applicable for samples, use any desired buffer without FBS or serum in buffer.
Note: Do not over trypsinize for adherent cells. LMO oligo labeling may affect membrane strength depending on cell types and over trypsinized cells may be broken during droplet generation.
Labeling:
- Add 20 µL of the mixture of anchor and DNA sample barcode into 180 µL of cell suspension and mix gently by pipetting.
- Incubate for 5 minutes on ice.
- Add 20 µL of Co-Anchor solution and mix gently by pipetting.
- Incubate for 5 minutes on ice.
- Add 1 mL of 1% BSA in PBS (-).
- Pellet cells by centrifugation. Note: Do not centrifuge at high speed.
- Wash cells with 1% BSA in PBS at least twice by centrifugation.
- Resuspend cells in appropriate amount of 1% BSA in PBS (-) for flow cytometry or appropriate buffer for single cell workflow.
- For flow cytometry, measure the FAM positive fraction to evaluate the labeling efficiency.
NGS library submission
The addition of MULTI-seq library at 1% molar ratio vs the cDNA library will provide sufficient barcode sequence alignment of the MULTI-seq Library.
For example, pool 0.5 µL of the 1 ng/µL MULTI-seq library with 49.5µL of the 2 ng/µL cDNA library for 400M reads format (MULTI-seq: cDNA=1:99). The DNA sample barcode sequence is located after the poly(dT) sequence from the read1 side (Figure 1F). The detailed protocol is published as supplementary data.
MULTI-seq Labeling Efficiency and Sample Stability
To quantify the labeling efficiency and stability in samples, human foreskin fibroblasts (HFFs), HEK293T cells and NIH3T3 cells were briefly labeled with FAM-labeled DNA sample barcode#1. As shown in Figure 2A, more than 98% of cells were efficiently labeled with DNA sample barcode by MULTI-seq LMO oligos. The labeling is stable at least for 2 hours on ice, while the labeling efficiency will decrease when the co-anchor is not used (Figure 2B).
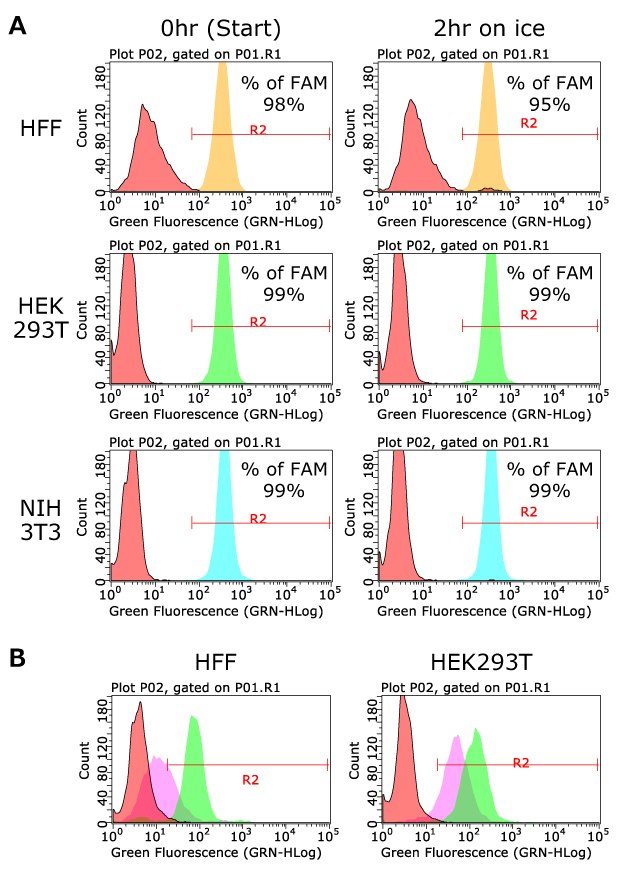
Figure 2.Labeling efficiency with FAM-labeled DNA sample barcode#1. A) Human foreskin fibroblasts (HFFs), HEK293 cells and NIH3T3 cells were labeled with LMO anchor and co-anchor containing FAM-labeled DNA sample barcode#1. Labeling efficiency was measured by flow cytometry. The GFP channel was used for capturing the FAM-positive cell population. B) Cells were labeled with both anchor and co-anchor (Green) or anchor only (Purple) containing FAM-labeled DNA sample barcode#1. Cells with no LMO oligos (FAM-labeled DNA sample barcode#1 only) were used as negative controls (Orange). After 1hr at room temperature, FAM positive cells were captured with flow cytometry. The labeling efficiency was decreased in the condition lacking the co-anchor, demonstrating that both oligos are necessary for efficient cell labeling.
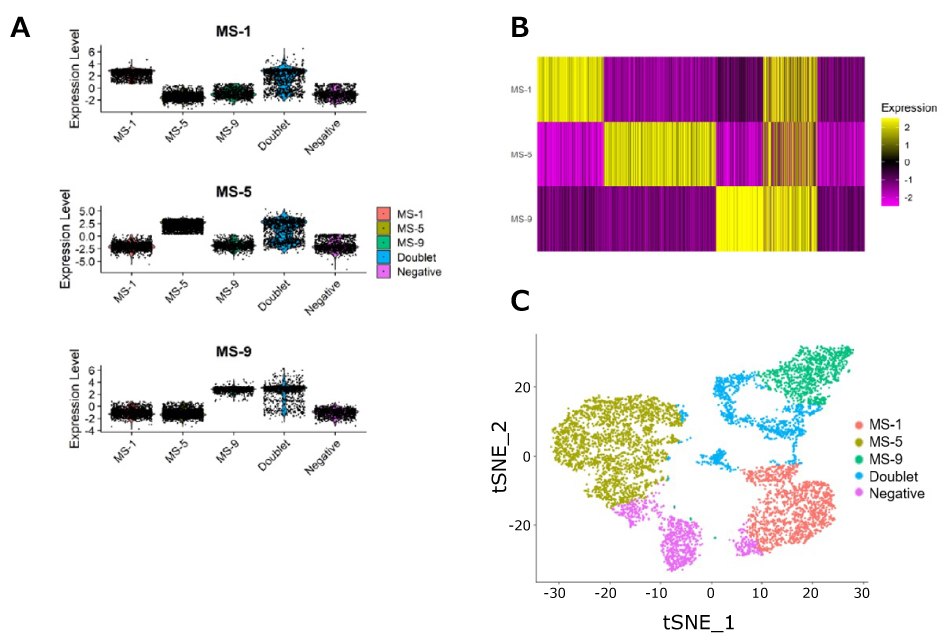
Figure 3.scRNA-Seq experimental sample data using MULTI-seq LMOs. PBMCs were isolated from three rhesus macaques and labeled with fluorescent antibodies (CD3/CD8/CD4). In parallel with antibody staining, each sample was labeled with a unique MULTI-seq sample barcode, following the manufacturer’s instructions. Samples were pooled, and CD8+ T-cells (CD3+/CD8+/CD4-) were sorted into 30uL RPMI + 10% FBS media using a FACS Aria. Cells were processed on a 10x Genomics instrument using 5´ GEX chemistry. Reads from the cell hashing library were processed using CITE-Seq-Count, and cell hashing calls were produced using an algorithm based on deMULTIplex. A) MULTI-seq labeling provides strong signal per cell, with clear separation over background. B) Heatmap using data from A demonstrates clear separation of samples and the ability to differentiate singlets, doublets, and negative cells. C) t-SNE was performed on the normalized count matrix, illustrating clear clustering by sample.
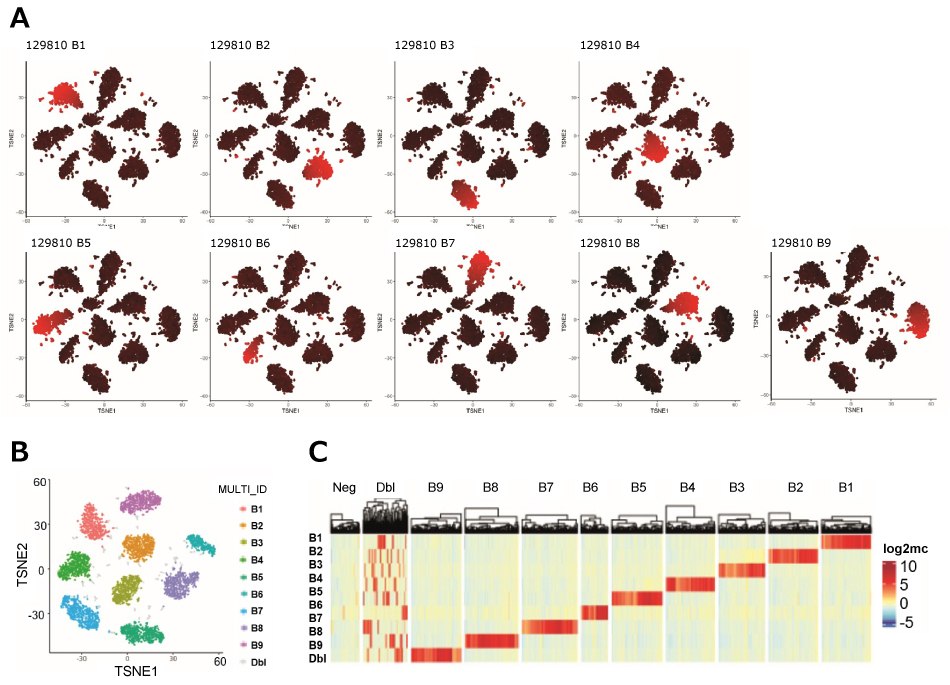
Figure 4.MULTI-seq demultiplexing and QC of a scRNA-seq experiment using 9 different barcodes. Raw barcode reads were log-2 transformed and mean-centered and then processed using the MULTI-Seq classification suite. A) Presence of each barcode was visually inspected by performing t-SNE on the normalized barcode count matrix. B1~B9 correspond to each barcode used. Color indicates barcode UMI abundances with low values in black and high values in red. B) t-SNE plot visualizing barcode classification of cells, excluding negatives. C) Heatmap showing cell barcode o sample barcode classification based on the McGinnis protocol (Ref 1) as row annotations.
MULTI-seq Troubleshooting Guide
- Issue: Droplets generation failed in MULTI-seq labeled cells.
Recommendation: Do not over trypsinize for adherent cells. LMO oligo labeling may affect membrane strength depending on cell types and over trypsinized cells may be broken during droplet generation. Alternatively, use a minimum amount of MULTI-Seq reagent. In this protocol, the minimum amount sets up to use 20 µL of 2 µM of each oligos for 500,000 cells. However, it is possible to reduce the amount of oligos depending on samples. We recommend setting up a minimum amount with the FAM-labeled DNA sample barcode#1 for sensitive samples. - Issue: Low amount of MULTI-seq DNA sample barcode library:
Recommendation: Use more template DNA sample barcode for PCR (up to 7~14 ng barcode DNA instead of 3.5 ng). - Issue: Small bands are co-amplified in MULTI-seq sample barcode library (It will be observed when more template is used for PCR):
Recommendation: Repeat 1.6X SPRI purification.
Products
References
To continue reading please sign in or create an account.
Don't Have An Account?