Ion Chromatography for Sugar Testing in Food and Environmental Analysis
Carbohydrates constitute the biggest part of the biomass on Earth. They are produced by photosynthesis and are present in all plants and plant-based materials. The amount and composition of carbohydrates in a sample can reveal a wide range of information, depending on the context. As a result, they are subject to analysis in various industries. In this article we demonstrate how ion chromatography is well-suited as an analytical technique for sugar analysis.
Carbohydrates are Everywhere
In the food industry, carbohydrate and sugar content are notable for being key factors in determining the nutritional value of food and drink. In environmental analysis—to mention but one example—the anhydrosugar levoglucosan, which is produced by the pyrolysis of cellulose and acts as a tracer for biomass combustion, is determined in aerosols. These are just two of the many applications of carbohydrate analysis. Carbohydrates are composed of one or more monosaccharide units, each of which has a carbonyl group (aldehyde or ketone group) and several hydroxyl groups.1 Because mono-, di-, and oligosaccharides are water-soluble, ion chromatography, which is performed in the aqueous phase, is particularly suitable for their analysis. It does not require extraction to the organic phase; thus, determination can be performed directly. However, a high-capacity column is necessary because sugars are relatively large molecules which are in many cases similar in structure (e.g., glucose and galactose; Figure 1).

Figure 1. Structural formulae of glucose and galactose. The molecules differ only in the position of the OH group at the C4 atom (highlighted with an asterisk).
Sugars in Foods
Since December 2016, the European Union (EU) requires that nutritional values are indicated on all foodstuffs, with the exception of unprocessed products and products sold loose (regulation no. 1924/2006). What is already established practice, i.e., indicating the calorific value and certain nutrients, including sugar and carbohydrates, is set to become mandatory.
Along with starch, which is a polymer of glucose, the usable carbohydrates found in foodstuffs are largely in the form of sugars. According to the EU definition, this includes all mono- and disaccharides with the exception of polyvalent alcohols. The majority of sugars in foodstuffs are made up of the monosaccharides glucose, fructose, galactose, and the disaccharides sucrose, lactose, and maltose.
Apple Juice Analysis
The chromatogram in Figure 2 was taken after the injection of apple juice, which was diluted (1:1000) with ultra-pure water. Apart from that, no sample preparation is necessary. The alkaline eluent (100 mM sodium hydroxide/10 mM sodium acetate, Cat. No. 78348) ensures that the sugars are present in dissociated form (as anions) and can therefore be separated in the column using the ion exchanger.
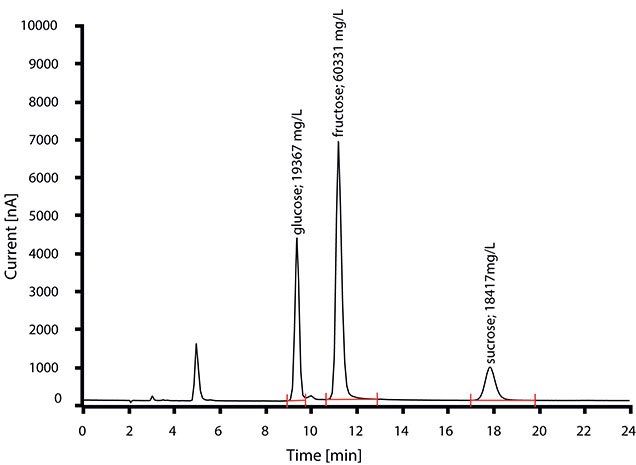
Figure 2.Determination of glucose, fructose, and sucrose in apple juice. Except for simple dilution, no sample preparation is required.2
Because carbohydrates are electrochemically active, they can be detected amperometrically. During amperometric detection, the analytes are oxidized to a working electrode by applying a potential to the latter. This results in an electrical current that reveals the concentration. Over time, however, carbohydrates form deposits on the working electrode when a continuous potential is applied. The amperometric detector is therefore operated in PAD mode (pulsed amperometric detection). Here, a three-stage cyclic potential ensures that after measuring the current, i.e., after the determination stage, the electrode is cleaned from the adsorbed molecules and eventually conditioned.
Residual Lactose in ‘lactose-free’ Products
A key part of the quality control of products declared lactose-free is the determination of residual lactose. The ion chromatogram in Figure 3 illustrates the determination of lactose in ‘lactose-free’ milk to which 100 mg/L lactose was added. Again, the separation takes place under strongly alkaline conditions (eluent of 5 mM sodium hydroxide/2 mM sodium acetate) and the analyte is detected by pulsed amperometry.
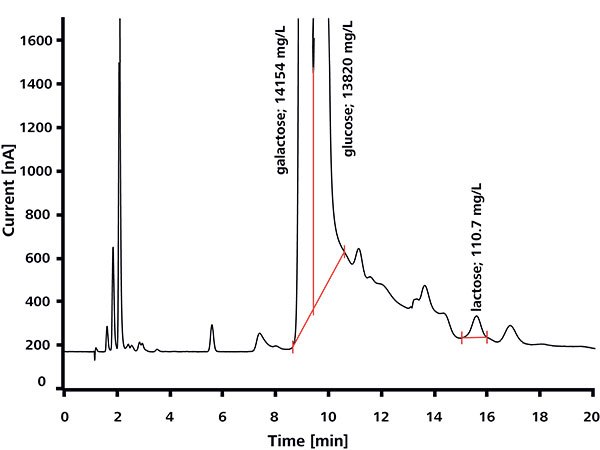
Figure 3. Determination of lactose traces in milk declared lactose- free, spiked with 100 mg/L lactose.3
The high concentrations of galactose and glucose illustrated in the chromatogram are a result of the enzymatic breakdown of lactose into these very monosaccharide constituents (Figure 4). Because of its protein-rich matrix milk must undergo dialysis before being analyzed, with the Metrohm Inline Sample Preparation. This is a fully automated process, and therefore does not involve any additional effort.
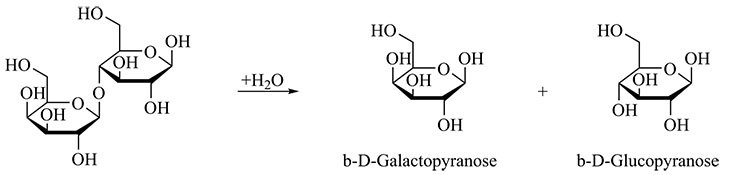
Figure 4. Lactose is composed of the monosaccharides galactose and glucose. The hydrolysis of lactose illustrated here is catalyzed by the enzyme lactase.
Carbohydrates as Tracers in Environmental Analysis
Fine dust limit values, which are used as health protection measures, are regularly being violated in many places. When looking for the culprit, the usual suspects are traffic and industry, but residential wood burning used for heating has also been linked to high fine dust values.4 The tracer levoglucosan (Figure 5) is often determined in order to detect wood combustion.
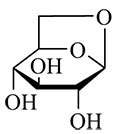
Figure 5. Levoglucosan (1,6-Anhydro-β-D-glucopyranose) is produced in the pyrolysis of cellulose and is therefore commonly used as an indicator for biomass combustion.
In Figure 6 the determination of a standard solution is shown, in which levoglucosan, mannosan, and galactosan—all products of wood combustion—were analyzed, as were several biological sugars, alcohols, etc., which are typically found on aerosol particles. The high-capacity column achieves good separation of all substances, which then can be determined in a single analysis.

Figure 6. Determination of indicators for wood combustion (levoglucosan, mannosan, and galactosan) and biological sugars and alcohols, which are found in aerosols such as pollen.5
The new "Carbohydrate Column"
The Metrosep Carb 2 chromatography column excels with its high ion exchange capacity, i.e., with the high number of ion exchange groups contained in its carrier material. This allows clean separation of the various sugars. Applications are found in a wide range of industries: water and environmental analysis, the pharmaceutical and food industry, forensics, the cosmetic industry, and the quality control of biofuels. In addition to carbohydrate analysis, the Metrosep Carb 2 is also suitable for determinations in samples with high salt content where lower-capacity columns fail, e.g., seawater.
For best performance, we have developed the alkaline IC eluent (Cat. No. 78348) for the Metrosep Carb 2 column. We also offer a representative range of carbohydrate certified reference materials (CRM) solutions for IC.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?