Determination of Ascorbic Acid and Dehydroascorbic Acid in Different Food Products and Supplements - A Simple HPTLC Based Approach
Introduction
Vitamin C or ascorbic acid is a vitamin found naturally in many fruits and some vegetables or is added to certain processed foods or dietary supplements. It is water-soluble and has antioxidative capacities by degrading to dehydroascorbic acid. The human organism cannot produce ascorbic acid and it must be ingested through food or supplements. It has essential functions in human body and maintains numerous vital processes. When vitamin C is deficient (scurvy), symptoms can occur such as fatigue, tiredness, and inflammations. The recommended daily dose of ascorbic acid is about 100 mg per day and can easily be reached with a healthy, balanced diet. Typically, ascorbic acid is quantified by iodometric titration according USP method.1 An additional substance identification is required and performed by e.g. infrared analysis.1
In the following application, we show an easy and fast screening approach for the simultaneous quantitative analysis of ascorbic acid and dehydroascorbic acid by High Performance Thin Layer Chromatography (HPTLC). Thin layer chromatography (TLC) and HPTLC are convenient, fast, and efficient separation techniques that enable the development of analytical methods without the need for extensive sample preparation or high investments.2 When combined with MS, a subsequent substance identification is possible. Low cost and short analysis time per sample are given by the parallel analysis of ascorbic acid in food products on one plate. Furthermore, the high matrix tolerance of TLC offers additional opportunities to existing routine methods. The high viscosity and high sugar content of many ascorbic acid products (e.g. fruit juice) makes them very complex and matrix-rich samples to analyze.
Experimental Conditions
Five different commercially available ascorbic acid containing products, like juice concentrate, fruit gums, vitamin C effervescent tablet, multi vitamin effervescent tablet, and a tablet with cranberry extract were analyzed using conditions in Table 1.
Due to its oxidative capacity, ascorbic acid gets rapidly decomposed and dehydroascorbic acid is formed. A reliable quantification of ascorbic acid can be challenging and requires a gentle but rapid analysis of the samples. In practice, ascorbic acid might be quantified together with a low amount of its dehydration product dehydroascorbic acid. (Figure 1). To simulate this effect, in this experiment, standards of ascorbic acid were over-spotted with dihydroxyascorbic acid.

Figure 1.Chemical structure of ascorbic acid and dehydroascorbic acid.
Calibration curves of ascorbic acid and dehydroascorbic acid were established based on 3 different applied standard volumes (Table 2). After separation, a fast and simple substance confirmation by MS was performed.3
The samples and standards were applied bandwise (6.0 mm). At first, the concentration series of the ascorbic acid standards were applied and afterwards over-spotted with the dehydroascorbic acid standards. Due to expected lower concentration of dehydroascorbic acid, the sample volume was lower than for ascorbic acid.
The plate was developed with the mobile phase and afterwards dried at 50 °C until completely dry. To quantify, the plate was heated at 110 °C for 10 minutes. Examination of the plate was done at 366 nm.
Results and Discussion
At 366 nm illumination, ascorbic acid appears at hRf 45 and dehydroascorbic acid at hRf 58 (Figure 2). MS measurement of the spots (before heating) were carried out to confirm substance identification.3
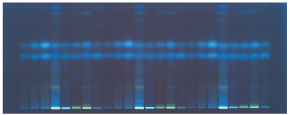
Figure 2.Visualization of the plate under UV 366 nm. Ascorbic acid appears at hRf 45 and dehydroascorbic acid at hRf 58.
The calibration solution profiles (Table 2) at 366 nm were used for establishing the calibration curves and quantification (Table 3, Figure 3 & 4) related to amount applied to the plates.

Figure 3.Calibration plot with corresponding calibration function of ascorbic acid.

Figure 4.Calibration plot with corresponding calibration function of dehydroxyascorbic acid.
The calibration data was used to quantify the vitamin C content of the five applied samples. In all 5 samples ascorbic acid and also dehydroascorbic acid could be determined. The results are displayed in Table 4 a & b.
Conclusion
The developed application procedure provides a simple method based dehydroascorbic acid and ascorbic acid analysis in different kind of samples and matrices by HPTLC. This easy and straightforward approach represents an alternative method for a reliable screening of vitamin C in food & beverage samples.
It provides a fast, economic, and simple semi-quantification of ascorbic acid and dehydroxyascorbic acid, and also demonstrates the main advantages of the TLC approach, such as quick sample preparation, high matrix tolerance, and high-throughput.
To find more on applications for food testing see SigmaAldrich.com/food.
Products
References
To continue reading please sign in or create an account.
Don't Have An Account?