Neonicotinoids Pesticides and Metabolites
Active Neonicotinoide
In spring 2008, a mass death of bees in Germany’s Baden-Württemberg state was reported. The German Federal Office of Consumer Protection and Food Safety (BVL) acted promptly, ordering the immediate suspension of the approval for eight seed treatment products used in oilseed rape (canola) and sweetcorn, which contained the following neonicotinoide pesticides: imidacloprid, clothianidin, thiamethoxam and methiocarb. According to the German Federal Research Centre for Cultivated Plants, 29 out of the 30 dead bees it had examined had been killed by contact with clothianidin. This was tied to a failure to apply a “glue” agent that affixes the compound to the coats of seeds. The manufacturer maintains that without the fixative agent, the compound drifted into the environment from sown rapeseed and sweetcorn, thus affecting the honeybees.
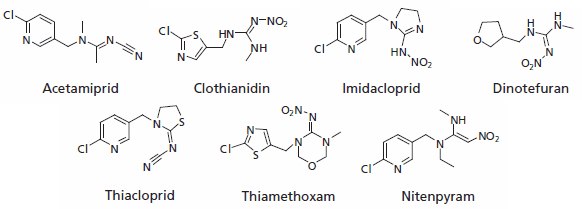
Figure 1.Neonicotinoids
Mode of action
Neonicotinoids are fairly new chemicals, but they have established themselves as key components in insecticides because of their unique selectivity. The mode of action of neonicotinoids is similar to the natural insecticide nicotine. They selectively bind and interact with the insect nicotinic acetylcholine receptor site. When neonicotinoids bind to the binding site of an insect, their electronegative tip, consisting of a nitro or cyano group, interacts with a unique cationic subsite of the insect’s receptor. On the other hand, the action of protonated nicotinoids requires a cationic interaction for binding to a mammal receptor. In insects, neonicotinoids cause paralysis which leads to death, often within a few hours; however, they are much less toxic to mammals, and under the WHO/EPA classification these compounds are placed toxicity class II or class III. Because the neonicotinoids block a specific neural pathway that is more abundant in insects than in warm-blooded animals, these insecticides are selectively more toxic to insects than mammals. This target site selectivity is a major factor in the favorable toxicological properties of neonicotinoids.
Metabolism
Methiocarb
Methiocarb does not belong to the group of neonicotinoide but is one of the ingredients of the banned seed treatment products. It is mainly used as an acaricide, as seed treatment for control of fruit flies on maize, flea beetles on oilseed rape, a molluscide and a bird repellent. It is a potent neurotoxin carbamate and acts as a cholinesterase inhibitor. The main metabolism pathways are cleaving of the carbonyl group and oxidation of the sulphur atom. If released to soil or water, methiocarb hydrolyses quite rapidly to
4-methylthio-3,5-dimethylphenol and methylcarbamic acid. The oxidation leads to methiocarb sulphone or methiocarb sulphoxide.
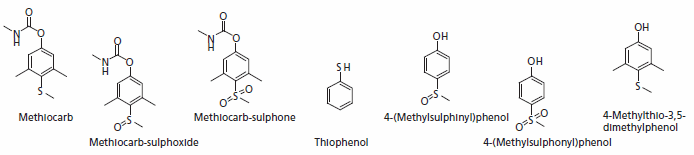
Figure 2.Major Metabolites of Methiocarb
Imidacloprid
Because of its low toxicity to most animals, other than insects due to its specificity for this type of nicotinic acetylcholine receptor, imidacloprid allows for lower concentrations to be used for insect control than other neurotoxins (particularly organophosphates). Used as a systemic insecticide, it is taken up by plant roots and diffuses in the plant via the xylem; its systemic properties then rely on insects ingesting the insecticide.
In the body the main metabolites are 6-chloronicotinic acid, 6-Hydroxynicotinic acid and 6-Methylmercaptonicotinic acid.
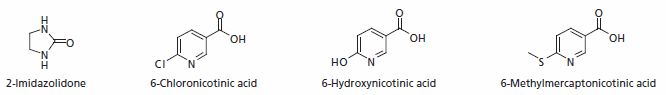
Figure 3.Major Metabolites of Imidacloprid
Clothianidin
Clothianidin is one of the newest neonicotinoids and a systemic insecticide. It is mainly used as a seed treatment. The main metabolites are 1-Methyl-3-nitroguanidine (MNG), nitroguanidine (NTG), N-(2-chlorothiazol-5- ylmethyl)-N-nitroguanidine (TZNG), N-(2-chlorothiazol- 5-ylmethyl)-N-methylurea (TZMU).
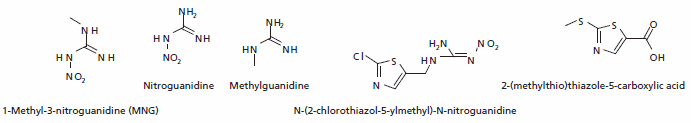
Figure 4.Major Metabolites of Clothianidin
Thiamethoxam
Thiamethoxam belongs to the new class of thianicotinyl compounds of the neonicotinoids (2nd generation). They possess a chlorothiazole heterocycle. Its mode of action in the plant is similar to the rest of this substance class. Its main metabolites are listed below. We are glad to offer our customers diverse deuterated and non-deuterated neonicotinoids in standard quality as well as several main metabolites.
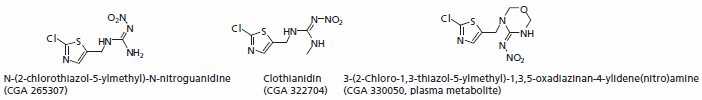
Figure 5.Major Metabolites of Thiamethoxam
To continue reading please sign in or create an account.
Don't Have An Account?