Antimicrobial Peptides
Chloe McClanahan
Read more about
With bacterial resistance and emerging infectious diseases becoming potential threats to humans, ribosomally synthesized antimicrobial peptides have become a promising focus area in antibiotic research. Antimicrobial peptides are classified as either non-ribosomally synthesized peptides or ribosomally synthesized peptides (RAMPs). Non-ribosomally synthesized peptides are found in bacteria and fungi. These antimicrobial peptides are assembled by peptide synthetases as opposed to ribosomal-supported synthesis. Gramicidin, bacitracin, polymyxin B, and vancomycin are examples of non-ribosomally synthesized antimicrobial peptides. These antibiotics are proven to be effective research tools, but compared to RAMPs they are disadvantageous for novel applications due to emerging bacterial resistance, for example vancomycin-resistant Staphylococcus aureus and enterococci.
RAMPs are derived from a diverse range of species, from prokaryotes to humans. Antimicrobial peptides comprise a host’s natural defense against the daily exposure to millions of potential pathogens. These peptides may also possess antiviral, antiparasitic, and antineoplastic activities. Over 500 RAMPs have been described in the literature. Their unique antibiotic spectrum is determined by amino acid sequence and structural conformation. RAMPs are gene-encoded peptides consisting of 12-50 amino acids with very little genetic overlap. A lack of sequence homology between RAMPs is indicative of evolutionary optimization of form and function in the species environment. RAMPs are typically cationic peptides with at least half of the amino acid residues being hydrophobic and a smaller number of neutral or negatively charged residues. Their amphipathic structure with opposing hydrophobic and lipohphilic faces aids in the perturbation of the bacterial cell wall.
The mechanism of action of a RAMP involves peptide binding to the bacterial cell surface, conformational change to the peptide, aggregation of multiple peptide monomers, and pore formation through the bacterial cell wall. RAMPs bind to lipopolysaccharides in the negatively-charged, Gram-negative bacterial outer cell wall or to the acidic polysaccharides of the Gram-positive bacterial outer cell wall. After binding, permeabilization of the bilayer membrane occurs by transient pore creation. Permeabilization leads to a leakage of cell components and cell death. There are several models of permeabilization although the precise mechanism is unknown. Three permeabilization models are termed barrel-stave, thoroidal, and carpet. Figure 1. depicts bacterial cell wall perturbation by a RAMP.
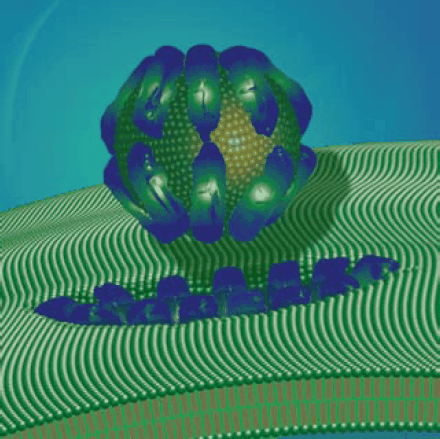
Figure 1.Antimicrobial peptide perturbation of the bacterial cell wall via the carpet model mode of action.
RAMPs are ideal candidates for clinical antimicrobial use because they:
1) Are active against antibiotic-resistant isolates
2) Do not select for resistant mutants and have limited natural bacterial resistance
3) Are synergistic with conventional antibiotics, specifically against resistant mutants
4) Are proven to kill bacteria in animal models
5) Kill rapidly
6) Provide benefical, supplementary activities, for example sepsis inhibition
Although RAMPs are ideal clinical candidates, their diverse structural variation makes it difficult to predict RAMP activity in vivo; therefore, it is challenging to design functional synthetic mimetics. Small changes in peptide sequence or conformation can lead to major in vivo differences in antimicrobial and cytotoxicity levels. An optimal in vitro minimum inhibitory concentration (MIC) against a range of bacterial organisms is 18 μg/mL. However, it is challenging to predict an ideal in vivo MIC from this in vitro MIC. In order to obtain MIC, specificity, stability and toxicity information, novel, synthetic antimicrobial peptides have been designed using data from RAMP-related, bioinformatic databases (Table 1). Production costs, protease susceptibility, and potential resistance from widespread use are additional concerns in the transition of RAMP application from a research to clinical setting.
Bacteriocins
Bacteriocins are non-pathogenic, antimicrobial peptides or proteins secreted by both Gram-positive and Gram-negative bacteria. Bacteriocins prevent the growth of similar bacterial strains but avoid damaging the host bacteria by selectively killing based on post-transcriptional modification and/or specific immunity mechanisms. Unlike the wide activity spectrum of conventional antibiotics, bacteriocins have a narrow activity spectrum. Additionally bacteriocins play a role in the regulation of signaling, virulence, and sporulation.
Nisin (Cat. No. N5764) is classified as a Class I, Type A lantibiotic. It is produced by Gram-positive, lactic acid fermentation bacteria and contains several atypical modified amino acids: thioetherbridged lanthionine, methyllanthionine, didehydroalanine and didehydroaminobutyric acid. Class I, Type A lantibiotics are elongated peptides that exhibit a range of activities including pore formation in bacterial bilayers while Class I, Type B lantibiotics are smaller, globular negatively charged or neutral peptides that inhibit specific enzymes. Class I, Type B lantibiotics include cinnamycin (Cat. No. C5241) and duramycin (Cat. No. D3168). An interesting subgroup of the non-lantibiotic bacteriocins is the Class IIa pediocin-like peptides. Pediocin (Cat. No. P0098) has been studied for its activity against pathogenic bacteria such as Listeria monocytogenes.
Although the genetic sequences of bacteriocins are not conserved, bacteriocin genes are often positioned near genes that aid in their production, for example transporter genes. BAGEL is a bacteriocin genome location tool developed and maintained by the Molecular Genetics Department at the University of Groningen, The Netherlands. This software is available for both academic and commercial use at http://bioinformatics.biol.rug.nl/websoftware/bagel/bagel_start.php.
Many of the bacteriocins are being studied for their application in food preservation. This methodology reduces requirements for potentially carcinogenic pesticides and heat treatments that reduce nutritional properties in food.
Bacteriocins may function as alternatives to conventional antibiotics that have been impacted by resistant strains. Millette, M. et al. recently demonstrated that nisin- and pediocin-producing bacteria reduced intestinal colonization by vancomycin-resistant Enterococci in vivo.
Insect RAMPs
Cecropin is a type of RAMP secreted within insects and active against Gram-negative bacteria. Cecropin A (Cat. No. C6830) is extracted from the hemolymph of the silk moth (Hyalophora cecropia) but has also been identified in porcine intestine. Antimicrobial peptides are often components of insect venoms, for example melittin from bee venom (Cat. No. M2272). It has been proposed that in primitive insect species RAMPs replace immune system processes, for example cytokine release, that characterize the bactericidal response in higher organisms. Drosophila synthesize different antimicrobial peptides in response to various infecting organisms. Kallio, J. et al. reported that RNAi targeting of several immune response genes in Drosophila caused altered antimicrobial peptide synthesis and identified involvement of the JNK signaling pathway in RAMP production.
Mammalian RAMPs
Although bacteriocins, insect, and mammalian RAMPs are similar in their bactericidal activity, the mammalian RAMPs also function as regulatory molecules in the host species’ immune response.
Defensins are a group of cationic, mammalian RAMPs that are commonly found on the skin, ear, epithelium, tongue, lung, and other surfaces frequently exposed to environmental pathogens. Phagocytic and epithelial cells, lymphocytes, and keratinocytes produce defensins. Figure 2 is an immunohistochemical staining of defensin-5 within respiratory epithelial cells using Prestige Antibodies® Anti-DEFA5 antibody, produced in rabbit (Cat. No. HPA015775). Defensins are constitutively expressed and stored in granules without external stimuli. However, increased levels of expression may be induced by proinflammatory cytokines, exogenous bacterial or LPS treatment.
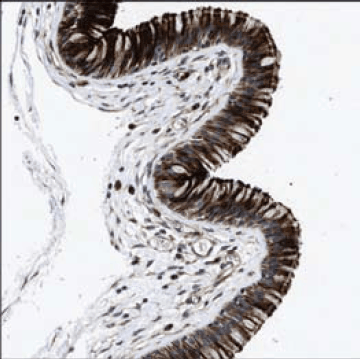
Figure 2.Immunohistochemical staining of human nasopharynx shows cytoplasmic and membranous positivity in respiratory epithelial cells using Prestige Antibodies® Anti-DEFA5 (Cat. No. HPA015775).
Like the bacteriocins, defensins consist of variable amino acid residue composition. The two classes of defensins are defined by structure. The human α-defensins have three intramolecular cysteine bonds whereas the larger β-defensins
(Cat. Nos. D9565, β-defensin 1 and D9690, β-defensin 2) consist of three antiparallel β-sheets and a unique disulfide bridge pattern connecting six cysteine residues. In addition to antimicrobial and antiviral activities, α-defensins inactivate LPS binding, regulate complement activation, and function as an adjuvant in mice. β-defensins induce prostaglandin production and play a regulatory role in the adaptive immune responses by functioning as chemoattractants for T lymphocytes as well as for immature dendritic cells via signaling through a chemokine receptor.
References
To continue reading please sign in or create an account.
Don't Have An Account?