Ionic Liquids
Introduction
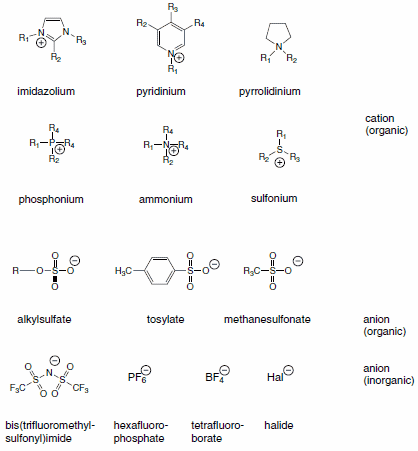
In this article, we highlight some applications of ionic liquids. We present over 50 additions to our portfolio of 130+ ionic liquids, ranging from well-known imidazolium and pyridinium derivatives to ammonium, pyrrolidinium, phosphonium, and sulfonium derivatives.

Ionic liquids are a class of purely ionic, salt-like materials that are liquid at unusually low temperatures. The definition of ionic liquids uses the boiling point of water as a point of reference: “Ionic liquids are ionic compounds which are liquid below 100 °C.” More commonly, ionic liquids have melting points below room temperature; some of them even have melting points below 0 °C. These new materials are liquid over a wide temperature range (300–400 °C) from the melting point to the decomposition temperature of the ionic liquid. Ionic liquids are generally considered to be safer alternatives to traditional organic solvents due to its low vapor pressures, that results in reducing the risk of inhalation exposure and air pollution, non-flammability, which decreases the risk of fire hazards compared to volatile organic solvents and biodegradability.
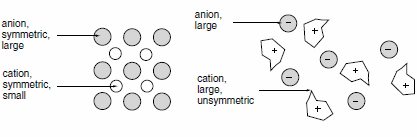
If we compare a typical ionic liquids, e.g., 1-ethyl-3-methylimidazolium ethylsulfate (m.p. <-20 °C), with a typical inorganic salt, e.g., table salt (NaCl, m.p. 801 °C), it becomes obvious why there is a difference. The ionic liquid has a significantly lower symmetry! Furthermore, the charge of the cation as well as the charge of the anion is distributed over a larger volume of the molecule by resonance. As a consequence, the solidification of the Ionic Liquid will take place at lower temperatures. In some cases, especially if long aliphatic side chains are involved, a glass transition is observed instead of a melting point.
The strong ionic (Coulomb-) interaction within these substances results in a negligible vapor pressure (unless decomposition occurs), a non-flammable substance, and in a high thermally, mechanically as well as electrochemically stable product. In addition to this very interesting combination of properties, they offer other favorable properties: for example, very appealing solvent properties and immiscibility with water or organic solvents that result in biphasic systems.
The choice of the cation has a strong impact on the properties of the ionic liquid and will often define the stability. The chemistry and functionality of the ionic liquid is, in general, controlled by the choice of the anion. The combination of a broad variety of cations and anions leads to a theoretically possible number of 1018 Ionic Liquids. However, a realistic number will be magnitudes lower. Today about 1,000 ionic liquids are described in the literature, and approximately 300 are commercially available. Typical structures combine organic cations with inorganic or organic anions:
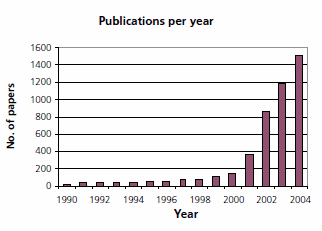
The growing academic and industrial interest in ionic liquid technology is represented by the yearly increase in the number of publications (source Sci-Finder™): starting from far below 100 in 1990 to more than 1,500 papers published last year.
Use and Applications
The reason for the increase in the number of publications can be attributed to the unique properties of these new materials. Ultimately, the possible combinations of organic cations and anions place chemists in the position to design and fine-tune physical and chemical properties by introducing or combining structural motifs and, thereby, making tailor-made materials and solutions possible. The following chart summarizes the important properties of ionic liquids and their potential and current applications:1
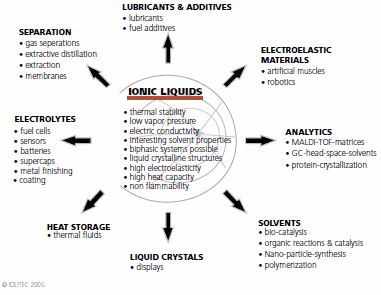
The corresponding applications of ionic liquids can be divided in their use as process chemicals and performance chemicals. In 1948, this class of electrically conducting materials (1-butylpyridinium chloride/AlCl3) first drew attention for their use as electrolytes for electrodeposition of aluminium.2 This field is still the subject of current research efforts. Other important applications in this context are their use as electrolytes for batteries and fuel cells.
The major breakthrough came with the advent of less corrosive, air-stable materials in 1992. Wilkes and Zaworotko introduced 1-ethyl-3-methylimidazolium tetrafluoroborate and focused on solvent applications.3 Although 1-butyl-3-methylimidazolium hexafluorophosphate and -tetrafluoroborate still dominate the current literature, there are better choices with respect to performance and handling. It was demonstrated in aqueous media the hexafluorophosphate anion and the tetrafluoroborate anion will degrade, resulting in the formation of HF, a noxious and aggressive acid.
Other interesting applications were suggested, derived from the unique combination of physical properties. For example, the use of ionic liquids as thermal fluids combines their heat capacity with thermal stability and a negligible vapor pressure. It is quite feasible to expect that many more applications will be brought forward by potential users and some of them will be realized in the future. To enable potential users to make use of Ionic liquids, it will be necessary to give technological support and to make a range of ionic liquids available for testing. This support also includes the development of toxicological data and government required notification as well as the implementation of recycling concepts for the ionic liquid.
References
To continue reading please sign in or create an account.
Don't Have An Account?