Chiral Phosphoric Acids: Versatile Organocatalysts with Expanding Applications
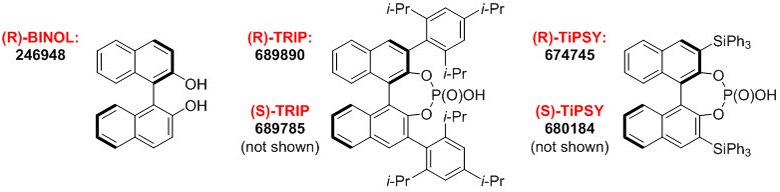
Figure 1.BINOL-derived Chiral Phosphoric Acids
Advantages
- Alternative to metal catalysts and chiral auxiliaries
- Both enantiomers of catalyst are available
- Relatively low catalyst loading (often 1-5 mol %)
- High selectivity at non-cryogenic reaction temperatures (-30 to 23 °C)
Representative Applications
Reductive Amination
One of the earliest demonstrations of chiral phosphoric acid catalysis is the metal-free reduction of imines with an organic reductant (Hantzsch ester) to give enantioenriched amines.1
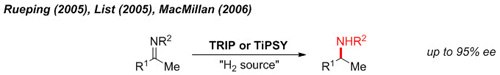
Figure 2.Reductive Amination
Allylation
The enantioselective allylation of aldehydes can be accomplished under very mild conditions at non-cryogenic temperature (-30 °C).2
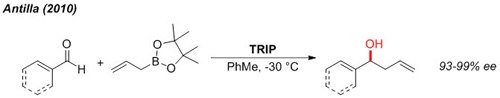
Figure 3.Allylation
Friedel-Crafts Alkylation
Functionalized indoles3,4 and pyrroles5 can be accessed in enantioenriched form by asymmetric alkylation.
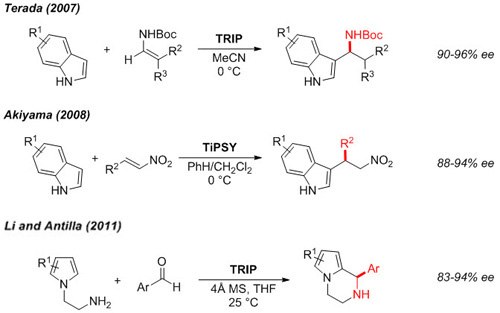
Figure 4.Friedel-Crafts Alkylation
Materials
Loading
References
1.
Rueping M, Sugiono E, Azap C, Theissmann T, Bolte M. 2005. Enantioselective Brønsted Acid Catalyzed Transfer Hydrogenation:? Organocatalytic Reduction of Imines. Org. Lett.. 7(17):3781-3783. https://doi.org/10.1021/ol0515964
2.
Hoffmann S, Seayad AM, List B. 2005. A Powerful Brønsted Acid Catalyst for the Organocatalytic Asymmetric Transfer Hydrogenation of Imines. Angew. Chem. Int. Ed.. 44(45):7424-7427. https://doi.org/10.1002/anie.200503062
3.
Storer RI, Carrera DE, Ni Y, MacMillan DWC. 2006. Enantioselective Organocatalytic Reductive Amination. J. Am. Chem. Soc.. 128(1):84-86. https://doi.org/10.1021/ja057222n
4.
Jain P, Antilla JC. 2010. Chiral Brønsted Acid-Catalyzed Allylboration of Aldehydes. J. Am. Chem. Soc.. 132(34):11884-11886. https://doi.org/10.1021/ja104956s
5.
Terada M, Sorimachi K. 2007. Enantioselective Friedel?Crafts Reaction of Electron-Rich Alkenes Catalyzed by Chiral Brønsted Acid. J. Am. Chem. Soc.. 129(2):292-293. https://doi.org/10.1021/ja0678166
6.
Itoh J, Fuchibe K, Akiyama T. 2008. Chiral Phosphoric Acid Catalyzed Enantioselective Friedel–Crafts Alkylation of Indoles with Nitroalkenes: Cooperative Effect of 3 Å Molecular Sieves. Angew. Chem. Int. Ed.. 47(21):4016-4018. https://doi.org/10.1002/anie.200800770
7.
He Y, Lin M, Li Z, Liang X, Li G, Antilla JC. 2011. Direct Synthesis of Chiral 1,2,3,4-Tetrahydropyrrolo[1,2-a]pyrazines via a Catalytic Asymmetric Intramolecular Aza-Friedel?Crafts Reaction. Org. Lett.. 13(17):4490-4493. https://doi.org/10.1021/ol2018328
Sign In To Continue
To continue reading please sign in or create an account.
Don't Have An Account?