Asymmetric Catalysis with Chiral Oxazaborolidinium Ions (COBIs): from Cyclization to Radical Reactions
Abstract
Chiral Lewis acid catalysts are some of the most powerful and efficient catalysts for asymmetric synthesis. Among the various Lewis acid catalysts known, the chiral oxazaborolidinium ion (COBI) has proven widely applicable to a variety of asymmetric transformations, such as the Diels–Alder reaction, nucleophilic additions, and cycloadditions. In this review, we introduce our recent work on COBI-catalyzed asymmetric reactions, including cyclopropanation, epoxidation, and radical reactions.
1. Introduction
Asymmetric synthesis is one of the most important types of organic synthesis, because the majority of therapeutic compounds and bioactive natural products exist in one enantiomeric form.1 The utilization of chiral auxiliaries or chiral reagents can be a good strategy for the asymmetric synthesis of chiral compounds,2 but suffers from the need to use stoichiometric amounts of the auxiliary or reagent. For this reason, asymmetric catalysis is an attractive and advantageous alternative for accessing enantiomerically enriched/pure compounds.3
Lewis acids have a long history of being employed as catalysts in the field of organic synthesis.4 However, their true power and relevance as highly efficient asymmetric catalysts have only been recognized in the past several decades. Consequently, a large number of chiral Lewis acid catalysts have been developed and cover almost all the metals in the periodic table.5
In particular, after the first report in 1976 of an enantioselective catalytic Diels–Alder reaction with a chiral boron complex,6 numerous chiral boron complexes have been designed and used extensively for catalyzing various cycloadditions, cyclizations, carbonyl reductions, and rearrangement reactions.7
The asymmetric reduction of prochiral ketones catalyzed by proline-derived chiral oxazaborolidines of type 1 was developed independently by Itsuno and Corey in 1981 and 1987, respectively (Scheme 1).8,9 This process, commonly referred to as the Corey–Itsuno reduction or Corey–Bakshi–Shibata (CBS) reduction, has proven effective in a large number of synthetic applications that have been reported in subsequent decades.10,11

Scheme 1.Corey–Itsuno or Corey–Bakshi–Shibata (CBS) Reduction catalyzed by chiral oxazaborolidines (1) . (Ref. 8,9)
Since it was first reported by Corey in 2002,12,13 the chiral oxazaborolidinium ion (COBI) has been used as a strong Lewis acid. COBI catalysts activated by Brønsted12,14 or Lewis acids,15—referred to as combined acid catalysts13c—exhibit higher catalytic activity and stereoselectivity than the individual acid catalysts through enhancement of their acidity by attachment of the Brønsted or Lewis acid. Representative structures of COBI catalysts that we discuss in this review are shown in Figure 1.
A large number of enantioselective Diels–Alder reactions were developed using cationic oxazaborolidines to produce enantioenriched cyclized products, and these results were reviewed in 2002 and 2009 by E. J. Corey.13a,b In addition, various asymmetric nucleophilic 1,2- or 1,4-additions to carbonyl compounds have been developed with COBI catalysts, and the results were reviewed in 2019 by our group.16 In this review, we highlight recent advances in the use of COBI catalysts to carry out the asymmetric formation of cyclic compounds (excluding Diels–Alder adducts) as well as enantioselective radical reactions.
All asymmetric reactions in this review have been shown to proceed via one of three pretransition-state assembly models of the COBI catalyst with carbonyl compounds (Figure 2).16 Thus, the stereochemistry of the asymmetric reactions can be rationalized based on one of these assembly models.
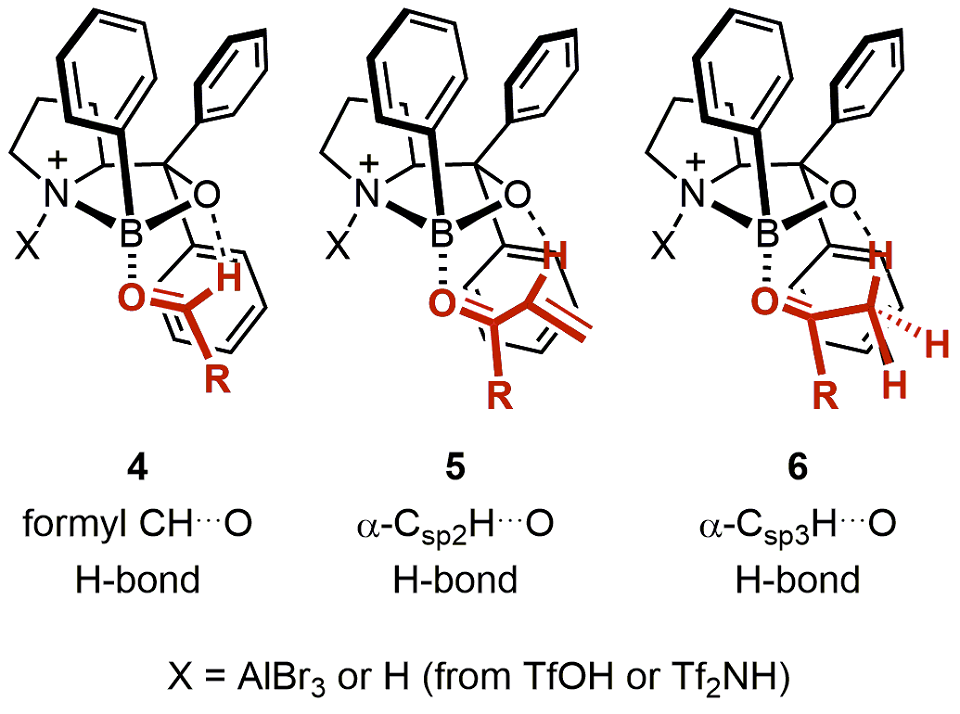
Figure 2.Pretransition-state assembly models for reactive complexes of COBI and carbonyl compounds. (Ref. 16)
Complexes of COBI with carbonyl compounds are proposed to possess a Lewis base–acid interaction between the carbonyl oxygen and boron atom of the COBI catalyst. Synergistically, the formyl CH…O (4) and α-CH…O (5 and 6) hydrogen bonding restricts the rotation of the bond between the boron atom and the carbonyl oxygen atom, which results in more rigid COBI-carbonyl complexes.17 In the pretransition-state models, the electron-deficient carbonyl carbon or the β-carbon of the α,β-unsaturated carbonyl compound is positioned above one of the geminal aromatic groups of COBI through a π–π donor–acceptor interaction. Moreover, the pseudoaxial aromatic ring of the catalyst effectively shields the rear face of the carbonyl compound from attack by nucleophiles and directs the addition of the nucleophile to the front face.
4. Conclusion
Asymmetric catalysis has been an important tool in organic synthesis. In particular, chiral Lewis acid catalysts have been applied to various synthetic methodologies and the total synthesis of natural products and pharmaceuticals. Among the chiral Lewis acid catalysts reported, chiral oxazaborolidinium ions (COBIs) are some of the most efficient catalysts for the activation of carbonyl compounds in such asymmetric reactions as dipolar cycloadditions, cyclopropanations, epoxidations, and visible-light-induced radical additions. We aimed in this review to, not only introduce various enantioselective methodologies that employ COBIs, but also provide a deeper understanding of the details of their catalytic action such as in pretransition state models or ternary EDA complexes. Further studies utilizing COBIs are underway in our laboratory to develop novel catalytic asymmetric methodologies for different types of nucleophilic addition reactions, rearrangements, radical reactions, and total syntheses.
5. Acknowledgments
We sincerely thank all the co-workers for their significant contributions to the research described in this review. We also acknowledge the National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIP) (No. RS-2023- 00219859 and 2022R1A6C101A751) and the R&D program of the institutional Research Program of KRICT (KK2332-10 and BSK23-415).
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?