1-hydrosilatrane
Introduction to 1-Hydrosilatrane
1-Hydrosilatrane (Figure 1) is a versatile hydride reagent and can be used in numerous reduction reactions to synthesize alcohols, amines, their chiral counterparts, and esters from aldehydes/ketones.1–6 The relative reactivity of 1-hydrosilatrane compared to other silanes, its stability to moisture, and its ease-of-handling make it an attractive reducing agent.
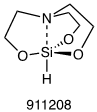
Figure 1. 1-Hydrosilatrane.
Silatranes were reported by Frye et al. in 1961 as the first examples of stable pentacoordinate alkoxysilanes.7 A fundamental feature of silatranes is that their caged structure encourages a dative bond between nitrogen and silicon.7–9 Hypercoordinate organosilanes are more electropositive at the silicon center, and thus, electron density increases at the ligands.10 Silatranes have various useful properties, however, they have not been widely used as reagents in organic synthesis.11
In 1976, Eaborn and co-workers were the first to investigate 1-hydrosilatrane as a hydride source, reporting one unoptimized example of the use 1-hydrosilatrane in the reduction of six different functional groups.12 Hydrosilanes are hydride sources as the Si-H bond is polarized towards the hydrogen.13 The intramolecular coordination between nitrogen and silicon in 1-hydrosilatrane activates the Si-H bond allowing it to be a potent hydride source.8,9 It is, however, highly stable due to its rigidity. Although this work provided preliminary evidence of the utility of 1-hydrosilatrane as a hydride reducing reagent, efficient synthetic methods using it have only recently (since 2016) been reported by the Adler group.1–6
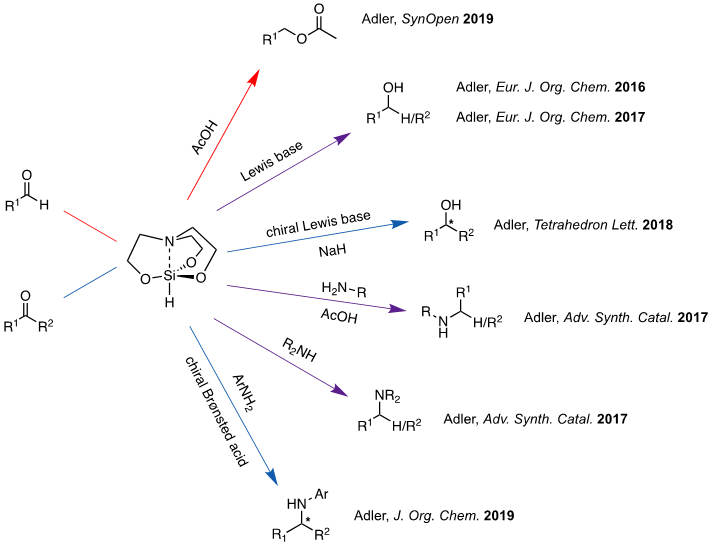
Figure 2. Methods utilizing 1-hydrosilatrane as a hydride reducing reagent
Benefits of 1-Hydrosilatrane Use
1-Hydrosilatrane is an air- and moisture-stable reducing agent that can be used in benchtop open-air reactions. It is non-toxic, easy to handle, and doesn’t degrade over time.2 It offers a safer alternative to metal hydride reductions and the methods are operationally simple and performed under mild conditions. Reductions with 1-hydrosilatrane are compatible with a variety of functional groups, show chemoselectivity, produce non-toxic by-products, are scalable, can be metal-free, and can be used to synthesize biologically important molecules and pharmaceuticals.
Representative Applications
1. Direct Reductive Amination
Direct reductive amination (DRA) represents the most commonly used method to synthesize secondary and tertiary amines.14 Methods for DRA must use a chemoselective hydride source to reduce the imine or iminium but not the starting carbonyl or other functionalities. 1-Hydrosilatrane is a relatively reactive reducing agent that is also compatible with various functional groups making it useful as a reducing agent in DRA.1,2 The DRA with aldehydes/ketones was developed using 1-hydrosilatrane showcasing its broad applicability to various aldehydes/ketones and secondary amines to make tertiary amines (Figure 3), with excellent functional group tolerance towards reducible and common protecting groups. Secondary amines were also synthesized with an aldehyde/ketone using acetic acid as the solvent (Figure 3). DRA ran with 1-hydrosilatrane also shows chemoselectivity towards aldehydes without reducing a ketone substituent on the same molecule. Thus, an amino alcohol could then be synthesized in a one-pot reduction with 1-hydrosilatrane (Figure 4).3

![1-hydrosilatrane-image-3-part-2 Direct reductive amination using 1-hydrosilatrane. [a] AcOH, r.t. [b] AcOH, 70˚C.](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/chemistry-and-synthesis/organic-reaction-toolbox/1-hydrosilatrane-image-3-part-2/1-hydrosilatrane-image-3-part-2.png)
Figure 3.Direct reductive amination using 1-hydrosilatrane. [a] AcOH, r.t. [b] AcOH, 70˚C.
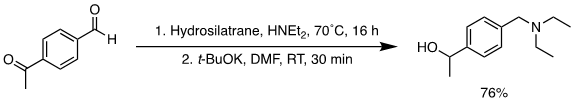
Figure 4.Two-step one-pot tandem DRA/reduction of a ketoaldehyde using 1-hydrosilatrane.3
Many important amines are chiral, which makes asymmetric DRA a vital transformation. To induce enantioselectivity, a bulky chiral phosphoric acid catalyst can be used in the DRA of prochiral ketones with arylamines using 1-hydrosilatrane as the reducing agent. Chiral amines are produced in high conversion and good enantiomeric excess (Figure 5).6
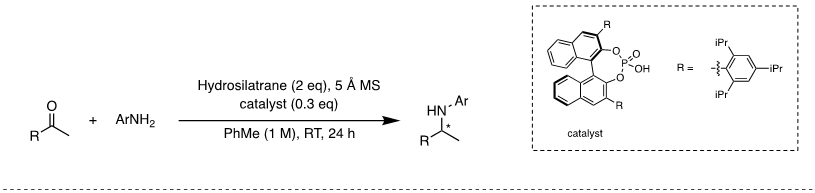
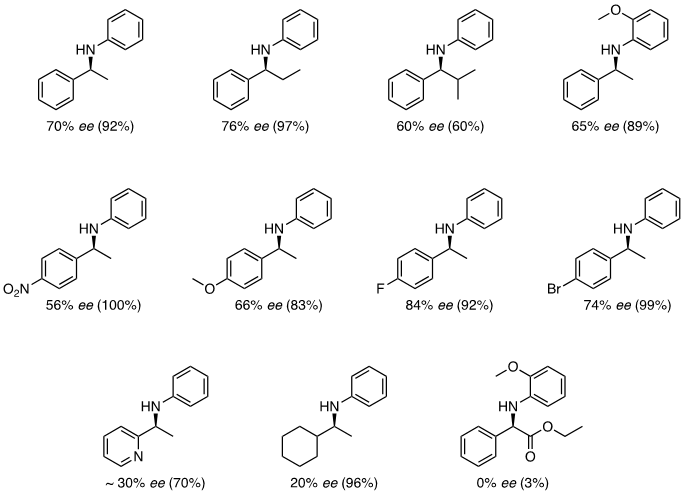
Figure 5. Asymmetric DRA using 1-hydrosilatrane and a chiral phosphoric acid catalyst.6
2. Reduction of Ketones
1-Hydrosilatrane is activated upon the addition of the Lewis basic tBuOK and can reduce various ketones into their corresponding alcohols under mild conditions without the production of volatile and hazardous silanes (Figure 6). Ketones can be reduced with high diastereoselectivity using 1-hydrosilatrane.2 Enantioselectivity was also demonstrated in the asymmetric reduction of ketones with the addition of a chiral additive to synthesize chiral alcohols in high enantiomeric excess
(Figure 7). 2,4

Figure 6.Reduction of ketones with 1-hydrosilatrane. 2
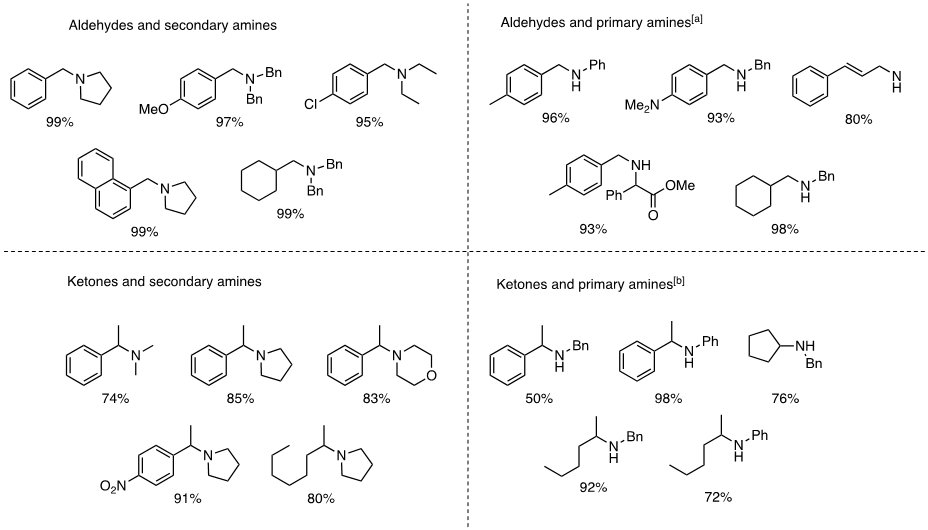
Figure 7.Asymmetric reduction of prochiral ketones with 1-hydrosilatrane and a chiral Lewis base.4
3. Reduction of Aldehydes
1-Hydrosilatrance can be used to reduce aldehydes into their corresponding primary alcohols. In this method, 1-hydrosilatrane is activated by NaOH and can reduce a wide range of aryl aldehydes into benzylic alcohols without generating ethers or deoxygenated products (Figure 8).1 No cross-reactivity was observed with potentially reducible nitriles, nitro groups, or benzyl/allyl ethers. Heteroaryl, polycyclic aryl, and aliphatic aldehydes have also been reduced by 1-hydrosilatrane efficiently.1

Figure 8.Reduction of substituted benzaldehydes.
Aldehydes can also undergo direct reductive acetylation to obtain acylated alcohols. This is useful for the synthesis of esters or the synthesis and in situ protection of alcohols, improving the efficiency of a multistep synthetic route. In the presence of acetic acid and an aldehyde, 1-hydrosilatrane is used as a reducing agent in the synthesis of various acetyl esters in good to excellent yields (Figure 9). This occurs without generating an ether by-product. Chemoselectivity is shown in this method towards aldehydes as a ketone was not reduced on the same molecule in exploring the substrate scope.5

Figure 9. Direct reductive acetylation of aldehydes.5
Special thanks to Melissa D’Amaral and Marc Adler for contributing this Technology Spotlight!
References
Materials
To continue reading please sign in or create an account.
Don't Have An Account?