Chiral Quest Phosphine Ligands
One of the most efficient methods for constructing chiral compounds is asymmetric hydrogenation. Catalytic asymmetric hydrogenations are among the most widely used industrial catalytic processes, due to their high turnover rates, efficiency and atom economy, and inexpensive material costs. Transition metal complexes associated with chiral phosphorus ligands are the dominant choice of catalysts for asymmetric hydrogenation, in large part due to the Nobel prize-winning, pioneering work of Noyori and Knowles. The requirement of an electron-rich chiral phosphine ligand is at the core of this transformation.
Professor Xumu Zhang at Penn State has made remarkable advances by creating a toolbox of chiral phosphines which can be used on a variety of substrates, some of which have been historically resistant to facile hydrogenation. Furthermore, an additional benefit in some reductions is reduced catalyst loading, due to increased turnover numbers (TON). Sigma-Aldrich is pleased to announce an agreement with Chiral Quest to distribute research quantities of a series of Zhang’s chiral phosphines for catalytic asymmetric hydrogenations.
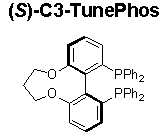
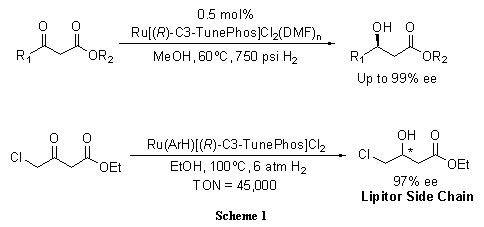
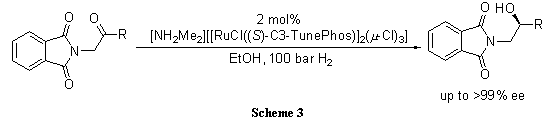
C3-TunePhos, a member of the atropisomeric aryl bisphosphine ligand family with tunable dihedral angles, provides comparable or superior enantioselectivities and catalytic abilities to BINAP in Ru-catalyzed asymmetric hydrogenation of ß-keto esters (Scheme 1),1 cyclic ß-(acylamino) acrylates (Scheme 2),2 and α-phthalimide ketones (Scheme 3).3

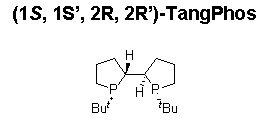
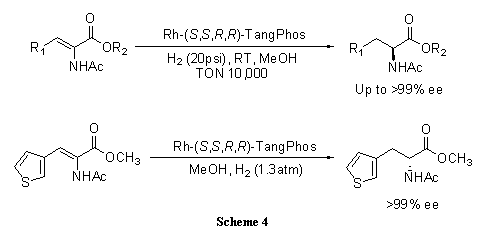
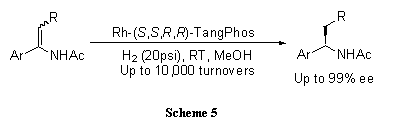
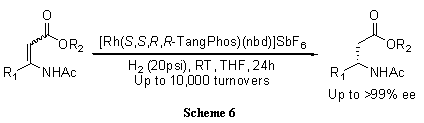
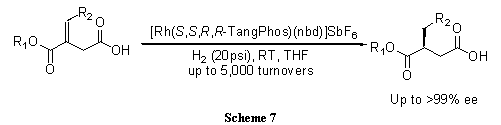
A highly electron-donating, low molecular weight, and rigid P-chiral bisphospholane ligand, TangPhos proves highly efficient in the rhodium-catalyzed hydrogenation of a variety of functionalized olefins such as α-dehydroamino acids (Scheme 4),4 α-arylenamides (Scheme 5),4 ß-(acylamino)acrylates (Scheme 6),5 itaconic acids (Scheme 7),6 and enol acetates (Scheme 8).6

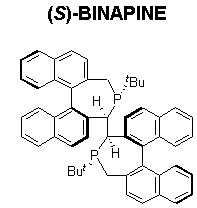
BINAPINE, a highly electron-donating rigid ligand, demonstrates excellent enantioselectivity and reactivity, with TON up to 10,000 for the asymmetric hydrogenation of Z-ß-aryl(ß-acylamino) acrylates (Scheme 9).7

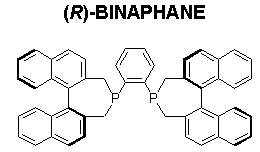
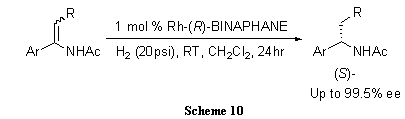
(R)-BINAPHANE shows excellent enantioselectivity (up to >99% ee) for hydrogenation of E/Z-isomeric mixtures of ß-substituted arylenamides (Scheme 10).8
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?