Electrochemical Allylic C–H Oxidation with N-Hydroxytetrachlorophthalimide (TCNHPI)
Introduction
Professor Phil Baran and coworkers have developed a new reagent, N-Hydroxytetrachlorophthalimide (ALD00564), which provides a cheap, scalable, and safe synthetic alternative to highly used transformations like allylic oxidations, as well as Negishi and Suzuki–Miyaura type cross-coupling reactions. In these cases, this reagent obviates the requirement for toxic reagents or precious metals, thereby lending itself to application on scale and in industrial applications.2
Advantages
- Redox-active reagent, since it readily accepts electron during various oxidative transformations1,3
- Due to the presence of electron-withdrawing groups (-Cl), it is superior to N-hydroxyphthalimide for use as a mediator in the synthesis of natural products2
- Replaces toxic reagents or expensive catalysts in several highly-used synthetic reactions, allowing its adoption on industrial scale
Representative Applications
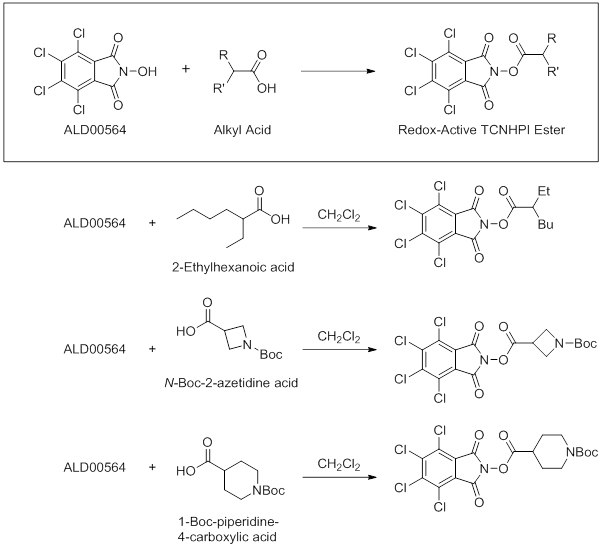
- TCNHPI (ALD00564) has been used in the synthesis of various redox-active esters via reaction with an alkyl acid.1,4 Listed below are some of the TCNHPI-derived, redox-active esters synthesized using TCNHPI in dichloromethane at room temperature.
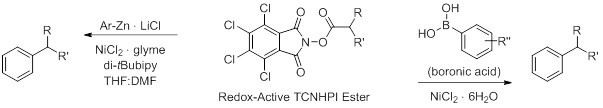
TCNHPI-derived esters readily undergo cross-coupling with aryl zinc reagents1 and are ideal coupling partners for Suzuki coupling reactions.4
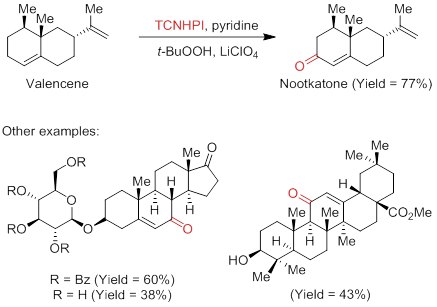
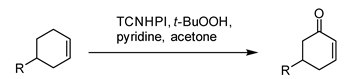
- TCNHPI can also be employed as a mediator in allylic C–H oxidation reactions useful for natural product synthesis, for which Baran and coworkers provide 40 examples, including steroid- and triterpene-derived compounds.2
- TCNHPI plays the role of a catalyst in various electrochemical allylic oxidation reactions. Due to its high oxidation potential (0.87 V versus Ag/AgCl), it effectively generates a higher-energy and more reactive tetrachlorophthalimido N-oxyl radical species during the oxidation reaction.2
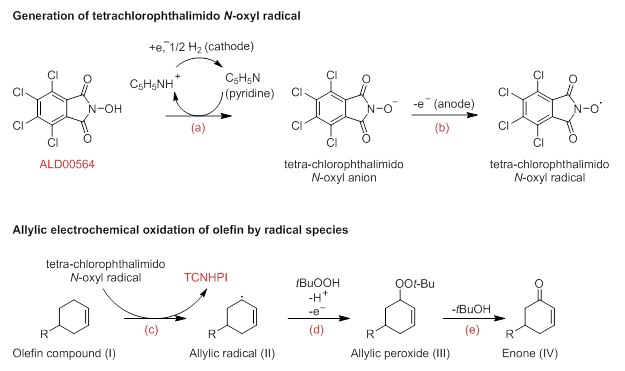
The proposed step-wise mechanism of the electrochemical allylic oxidation of an olefin by TCNHPI is described below:2
a) TCNHPI undergoes deprotonation with pyridine to afford tetrachlorophthalimido N-oxyl anion.
b) Anion undergoes anodic oxidation to afford tetrachlorophthalimido N-oxyl radical species.
c) Olefin compound (I) undergoes hydrogen abstraction, thus regenerating TCNHPI back and a stable allylic radical (II).
d) Allylic radical (II) reacts with electrochemically generated t-BuOO (from t-BuOOH) to afford allylic peroxide (III).
e) Removal of t-BuOH from (III) affords enone (IV).
Conclusions
In sum, ALD00564 is a cheap, scalable stoichiometric reagent that enables important transformations such as allylic oxidation as well as the Ni-catalyzed cross-coupling of carboxylic acids to boronic acids. These last are so well represented commercially that this technology (and reagent) will force a rethink in how molecules of all sizes and complexity are designed and synthesized.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?